Chapter 5 Periodic Classification of Elements Class 10 Science Notes
Students should read Chapter 5 Periodic Classification of Elements Class 10 Science Notes provided below. These notes have been prepared based on the latest syllabus and books issued by NCERT, CBSE and KVS. These important revision notes will be really useful for students to understand the important topics given in the chapter Periodic Classification of Elements in Class 10 Science. We have provided class 10 science notes for all chapters.
Revision Notes Chapter 5 Periodic Classification of Elements Class 10 Science
Chapter 5 Periodic Classification of Elements is an important chapter in Class 10 Science. The following notes will help you to understand and easily learn all important points to help you score more marks.
Important Terms & Concepts
1. Element:Substance containing atoms of one type only. Na ,K Fe ,Ca ,Mg, Cl etc.
There are 118 elements known to us .
2. Classification: Classification means grouping of elements on the basis of similarities in properties.
Need of Classification: It is difficult to study each and every element individually and to know its properties and uses.
Therefore, they have been classified into groups on the basis of their similarities in properties.
3. Basis of Classification: Classified is done on the basis of similarities in properties so that the systematic study could be made about them.
4. Earlier attempts at Classification: In 1803, Dalton published a table of relative atomic weights (now called atomic masses) which formed important basis of classification at that time.
5. Dobereiner Law of Triads: “When the elements are arranged in groups of three in increasing order of atomic masses, the middle element of a group has the atomic mass and properties roughly the average of the other two elements.”
These elements show similarity in their properties, e.g.,

Importance: The above classification of elements into triads had a great significance in predicting atomic mass and properties of middle elements. Even in these days, these days, these elements resemble in their properties.
Limitation: It failed to arrange all then known elements in the form of triads of elements having similar chemical properties.
6. Newland’s Law of Octaves: Newland’s arranged elements in order of increasing atomic mass.
It states ‘when elements are arranged in increasing order of atomic mass, the properties of the eighth element are kind of repetition of the first just like the notes of music, e.g.,

It is clear from the above table Li, Na and K resemble each other, Be, Mg and Ca resemble in properties, i.e., every eighth element resembles the first element.
7. Arrangement: The need of arranging element into vertical columns called groups and horizontal rows called periods thus became evident and in doing so, periodicity in their properties could be observed.
8. Limitation:
• All the elements at that time could not be classified into octaves.
• Applicable up to calcium that is for lighter elements.
• Properties of new element could not fit in it i.e., in some cases properties of elements were not same as defined by octave .
9. Periodicity of Properties: The repetition of similar properties after a definite interval is called the periodicity in properties .It is called periodic the elements are arranged on the basis of their atomic masses and also on the chemical p[properties.
10. Mendeleev’s Classification:
• Mendeleev arranged 63 elements known at that time on the basis of similarities in their observed that most of the elements were placed in the increasing order of their atomic masses.
• It was also observed that there is recurrence of elements with similar physical and chemical properties after a regular interval (periodic). That is, every eighth element had properties similar to that of the first.
• The two main features of Mendeleev’s periodic classification :Gaps in the periodic table ,and wrong order of atomic masses of some of the elements.
11. Mendeleev’s Periodic Law: It states “properties of the elements are periodic functions of their atomic masses.”

12. Groups: The vertical columns of the periodic table are called ‘Groups’.
13. Periods: The horizontal rows of the periodic table are called ‘Periods’.
14. Advantages of Mendeleev’s Periodic Table:
I) He could classify all the 63 elements discovered at that time on the basis of similarities in properties.
II) He left gaps for yet to be discovered elements.
III) He predicted the properties of undiscovered elements and thus helped in the discovery of these elements later on.
IV) He named them by prefixing a Sanskrit numeral eka (one), divi (two), tri (three), etc. to the name of the preceding similar (analogous) element in the same group, e.g., eka-boron, eka-aluminium, eka-silicon, ekamanganese, divi-manganese and eka-tantalum.
All these elements were discovered later and did have properties similar to those predicted by Mendeleev, e.g.,

It helped in correction of atomic weights of certain elements on the basis of their position in the periodic table.
15. Limitation of Mendeleev’s Periodic Table:
I) Although most of the elements were placed in the order of increasing atomic masses, increasing order could not be maintained in all cases, e.g., Cobalt (Atomic mass 58.93) preceded nickel (58.71): tellurium (127.6) preceded iodine (126.90) but he could maintain similarity in properties, e.g., ‘Te’ resembles ‘Se’. ‘I’ resembles ‘Br’.
II) The position of Isotopes could not be explained.
III) Mendeleev’s Periodic Table did not provide place for noble gases which were discovered later.
16. Modern Periodic Table: Henry Moseley, an English physicist found that the atomic number (Z) was the fundamental property of an element and not the atomic mass for classification of elements.
The real significance of the modern periodic classification based on atomic numbers is that it relates the periodicity in the properties of elements to the periodicity in their electronic configuration.
17. Modern Periodic Law: “Properties of elements are periodic functions of their atomic numbers, i.e., the number of protons or electrons present in the neutral atom of an element.”
18. Long form of Periodic Table: Elements are arranged in increasing order of their atomic numbers. The prediction of properties of elements and their compounds can be made with precision.
All drawbacks of Mendeleev’s Periodic Table vanish when the elements are arranged on the basis of increasing atomic numbers.
19. Elements in a Group:
I) They show similar chemical properties due to similar outer electronic configuration, i.e., same number of valence electrons.
II) They have gradation in properties due to gradually varying attraction of the nucleus and the outer valence electrons as we go down the group.
20. Main Features of the Long form of the Periodic Table:
I) It shows arrangement of elements bases on modern periodic law.
II) There are 18 vertical columns known as groups.
III) There are 7 horizontal rows known as periods.
IV) Elements having similar outer electronic configuration, i.e., having same valence electrons have been placed in same groups, e.g.,
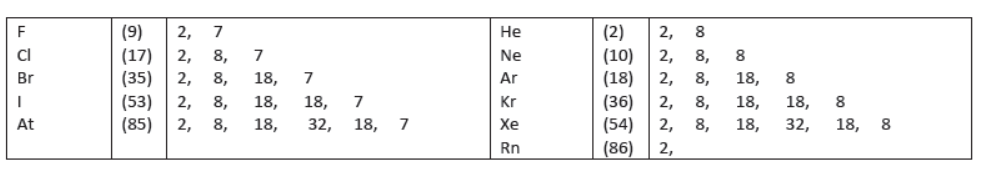
V) In the modern periodic table metals have been separated from non-metals by some elements called ‘Metalloids’ which are placed diagonally in the periodic table .ex As , Sb ,Te ,Po
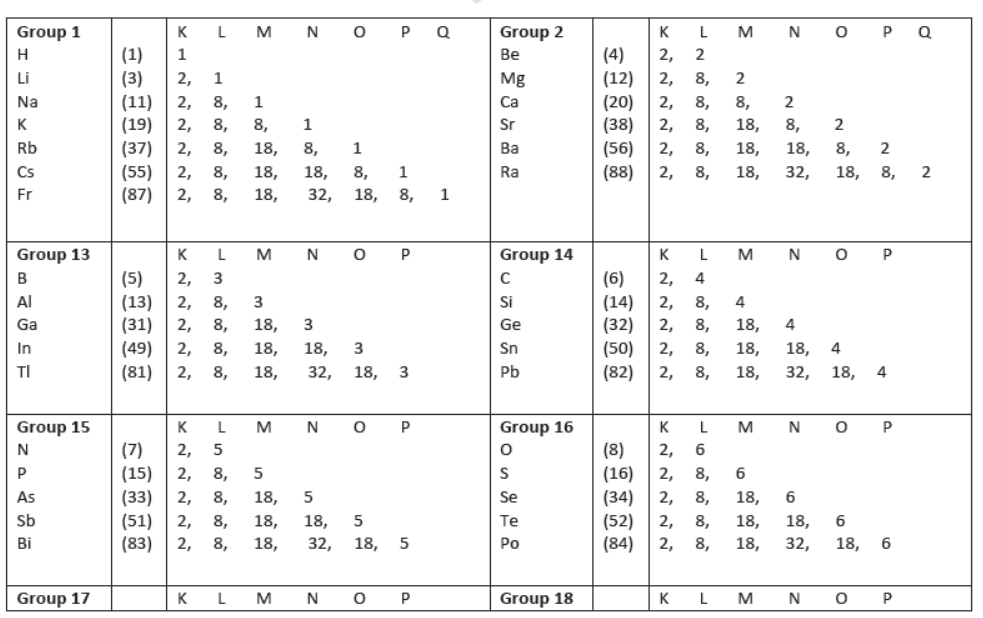
VI) In periods, the number of electrons in the outermost shell of the elements increases gradually, e.g.
Period 1 H He
(K-shell) 1 2
Period 2 Li (3) Be (4) B (5) C (6) N (7) O (8) F (9) Ne (10)
(K, L shells) 2, 1 2, 2 2, 3 2, 4 2, 5 2, 6 2, 7 2, 8
VII) Each group in the table signifies identical outer shell electronic configuration, i.e., same valence electron, e.g.,
group 1 has 1 valence electron, group 2 has 2 valence electron, group 13 has 3 valence electron, and group 14
has 4 valence electrons.
VIII) Each period starts with filling of new shell, e.g.,
1st Period —K shell (1st shell) starts filling with hydrogen (1) and ends at Helium (2).
2nd Period —L shell (2nd shell) starts filling from Li (3) upto Ne (10).
3rd Period —M shell (3rd shell) starts filling from Na (11) upto Ar (18).
4th Period —N shell (4th shell) starts filling from K (19) upto Kr (36) and so on.
IX) The periodic table is divided in four blocks:
a) s block elements − Group 1 and 2 elements are called s − block elements.
b) p block elements − Group 13 to 18 elements are called p − block elements.
c) d block elements− Group 3 to group 12 elements are called d − block elements or transition elements (in between s − block and p block− elements).
d) f block elements − The elements placed at the bottom of the periodic table are known as f − block elements. The fourteen elements after La (57) (Lanthanum) are called Lanthanoides and fourteen elements after Actinium Ac (89) are called Actinoides.
21. Limitations of Modern periodic table:
• Position of hydrogen is not justified.
• Lanthanoides amnd Actinoides are placed at the bottom
22. Naturally occurring Elements: Elements upto atomic number 92 occur in nature except Technetium, Tc (Z = 43) and Promethium, Pm (Z = 61) which are formed from radioactive elements where ‘Z’ represents atomic number.
23. Synthetic Elements: Elements beyond atomic number 92 are man-made elements. They are also called synthetic elements.
24. Metalloids (Semi metals)
They resemble with metals as well as non- metals
They are at border of metals and non-,metals
Ex –B ,Si ,Ge , Te are metyalloids.
25. Group:
Elements in a group have same number of valence electrons.
The chemical properties of elements in a group are similar due to same number of valence electrons, e.g., all the group 1 elements have 1 valence electron. They form positively charged ions by losing one electron. When required amount of energy is supplied to them i.e., Li2+, Na+, K+ .
Group 1 elements are called alkali metals.
Group 2 elements are called alkaline earth metals.
Group 2 elements have 2 valence electrons in the outermost shells. They can lose both the valence electrons to form dispositive cations, i.e.,Be2+, Mg2+, Ca2+ . Positively charged ions are called cations.
Group 13 elements belong to Boron family, Group 14 to Carbon family, Group 15 to Nitrogen family, Group 16 to Oxygen family.
Group 16 elements contain 6 valence electrons in their outermost shells, i.e., two electrons less than the maximum number of electrons that can be present in the outermost shell. They can gain 2 electrons more easily rather than lose 6 electrons. They change into dinegative ions such as O2-, S2-. Group 17 elements called Halogens contain 7 valence electrons. They can gain one electron to acquire stable electronic configuration, i.e., 8 electrons in the outermost shell and form uni-negative (single negative) ions such as F, CI-,Br, ,i– Negatively charged ions are called anions.
Group 18 elements called noble gases; have their outermost shell completely filled. The elements of this group have no tendency to lose or gain electrons. Thus, the elements of this group have zero valences and are almost unreactive.
Hence they are called Noble gases. However nowadays, compounds of Kr, Xe, and Rn have been prepared.
In any particular group, the number of shells increases but the number of valence electrons remains the same.
26. Periods:
I) The horizontal rows in the periodic table are called periods.
II) There are 7 periods in the long form of periodic table.
III) The first period contains 2 elements, Hydrogen and Helium. They have only one shell.
IV) The second period contains 8 elements:
Lithium Li (3), Beryllium Be (4), Boron B (5), Carbon C (6), Nitrogen N (7), Oxygen O (8), fluorine F (9) and Neon Ne (10). The second period has 2 shells (K and L) and L shell is progressively filled.
V) The elements of 3rd period are:
3rd Period Na (11) Mg (12) Al (13) Si (14) P (15) S (16) Cl (17) Ar (18)
(K, L, M shells) 2, 8, 1 2, 8, 2 2, 8, 3 2, 8, 4 2,8, 5 2, 8, 6 2,8, 7 2, 8, 8
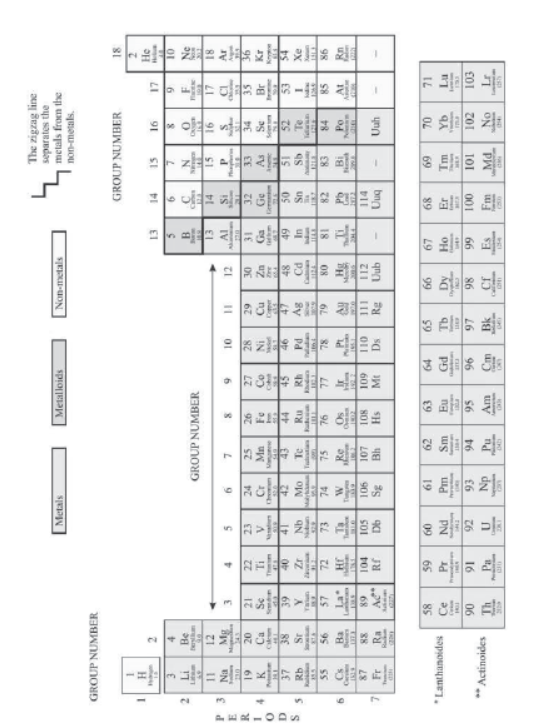
In 3rd period, there are three shells and 3rd shell (M-shell) is being progressively filled.
4th period has 18 elements
5th period has 18 elements
6th period has 32 elements
7th period has 32 elements. As on now 2011, 118 elements have been identified.
In periods, the number or valence electrons increases from left to right in s and P − block
27. Atomic Size (Atomic radii). Atomic size means radius of an atom. It is defined as distance between centres of nucleus an outermost shell (valence shell) of an isolated atom.
28. Covalent radii: It is defined as half of the distance between the centres of nuclei of two atoms (bond length) bonded by a single covalent bond, e.g., Bond length in case of H—H (Hydrogen molecule) is 74 pm.
Covalent radius 1/4 x 74pm = 37 pm (picometer) [1pm=10-12m]
It can be measured in case of diatomic molecules of non-metals.
29. Metallic Radii: It is defined as half of the internuclear distance between the two metal ions in a metallic crystal. It is measured in case of metals.
30. Variation of Atomic size in a Group: Atomic size generally increases from top to bottom in a group.
Reason: It is due to addition of a new shell, i.e., number of shells goes on increasing. e.g.,
pm stands for picometre, 10-12m i.e.,
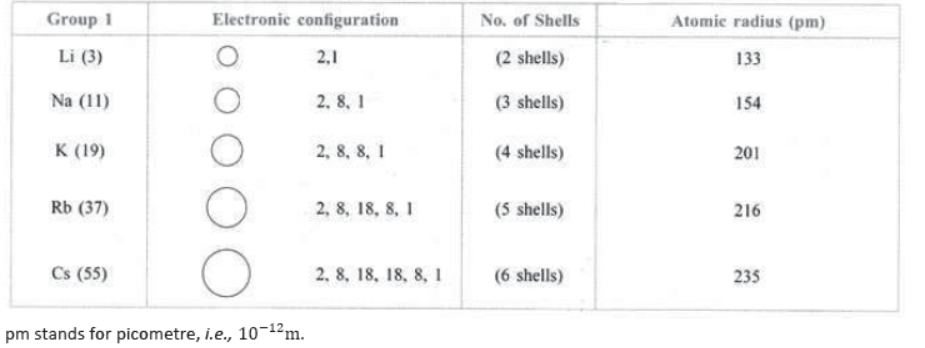
31. Variation of Atomic size along a Period: Atomic size goes on decreasing along a period from left to right.
Reason: It is due to increase in nuclear charge (number of protons in nucleus) which pulls the electrons towards it, i.e.,
force of attraction between nucleus and valence electrons increases, therefore atomic size decreases, e.g.,
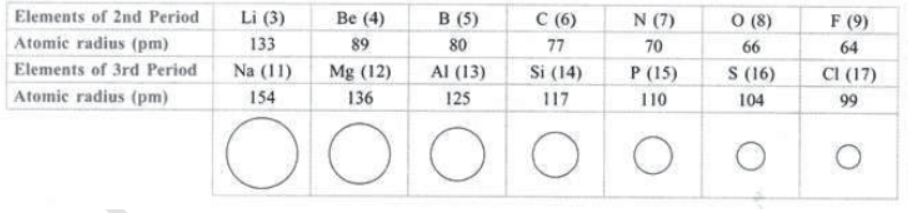
32. Tendency to Lose or Gain Electron: Chemical nature and reactivity of an element depend upon the ability of its atoms
to donate or accept electrons.
33. Variation of tendency to lose electron down a Group: Tendency to lose electron goes on increasing down the group.
Reason: It is due to increase in the distance between the valence electrons and the nucleus as the atomic size increases down a group, the force of attraction between the nucleus and the valence electrons decreases, therefore, tendency to lose electron increases down the group.
34. Variation of tendency to lose electron along a Period: It goes on decreasing generally along a period from left to right with decrease in atomic size.
Reason: Due to decrease in atomic size, the force of attraction between the valence electrons and the nucleus increases and, therefore, electrons cannot be removed easily.
35. Variation of tendency to gain electron down the Group: It goes on decreasing down the group in general.
Reason: Due to the increase in atomic size, the force of attraction between the nucleus and the electron to be added becomes less.
36. Variation of tendency to gain electron along a Period: It increases from left to right in a period.
Reason: It is due to decrease in atomic size which leads to an increase in the force of attraction between the nucleus and the electron to be added.
37. Metallic and Non-metallic Character: Group 1 to 12 are metals, Group 13 to 18 comprise non-metals, metalloids and metals.
Metalloids: Those elements which resemble both metals and non-metals are called metalloids. They are also called semi-metals, e.g., Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium and Polonium.
Properties of Metals:
I) They are malleable.
II) They are ductile.
III) They are good conductors of heat and electricity.
IV) They have generally 1 to 3 valence electrons.
V) They have the same or less number of electrons in their outermost shell than the number of shells.
VI) They are mostly solids.
Properties of Non-metals:
I) They exist in solid, liquid or gaseous state.
II) Non-metals are generally brittle.
III) They are nonconductors.
IV) They have 4 to 8 valence electrons.
38. Variation of Metallic Character: Metallic character increases down a group due to increase in tendency to lose electron. It decreases along a period due to decrease in tendency to lose electrons.
39. Variation of Non-metallic Character: Non-metallic character decreases down a group because of decrease in tendency to gain electron which is due to increase in atomic size.
Along a period, non-metallic character increases from left to right due to increase in tendency to gain electron which is due to decrease in atomic size.
40. Reactivity of Metals: It increases down the group due to increase in atomic size therefore, they can lose electrons easily.
41. Reactivity of Non-metals: It decreases down the group as atomic size increases and tendency to gain electrons decreases.
Periodic Classification Of Elements Class 10 Science Important Questions
Question. The positions of four elements A, B, C and D in the modern periodic table are shown below. (Image 106)
Which element is most likely to form an acidic oxide?
(a) A
(b) B
(c) C
(d) D
Answer
C
Question. In Mendeleev’s periodic table, gaps were left for the elements to be discovered later. Which of the following element found a place in the periodic table later?
(a) Germanium
(b) Chlorine
(c) Oxygen
(d) Silicon
Answer
A
Question. An element X is forming acidic oxide. Its most probable position in the modern periodic table is:
(a) Group 1 and Period 3
(b) Group 16 and Period 3
(c) Group 17 and Period 3
(d) Group 2 and Period 3
Answer
C
Question. Arrange the following elements in the order of their decreasing metallic character Na, Si,Cl, Mg, Al:
(a) Cl > Si > Al > Mg > Na
(b) Na > Mg > Al > Si > Cl
(c) Na > Al > Mg > Cl > Si
(d) Al > Na > Si > Ca > Mg
Answer
B
Question. Which among the following elements has the largest atomic radii?
(a) Na
(b) Mg
(c) K
(d) Ca
Answer
C
Question. Which of the given elements A, B, C, D and E with atomic number 2, 3, 7, 10 and 30 respectively belong to the same period?
(a) A, B, C
(b) B, C, D
(c) A, D, E
(d) B, D, E
Answer
B
Question. On the basis of electronic configuration ofX59, the group number and period of the element ‘X‘ is:
(a) Group 15 period 2
(b) Group 13 period 2
(c) Group 9 period 5
(d) Group 13 period 5
Answer
D
Question. The elements A, B, C, D and E have atomic number 9, 11, 17, 12 and 13 respectively.
Which pair of elements belongs to the same group?
(a) A and B
(b) B and D
(c) A and C
(d) D and E
Answer
C
Question. An element ‘X‘ with atomic number 11 forms a compound with element ‘Y‘ with atomic number 8. The formula of the compound formed is:
(a) XY
(b) XY
(c) XY2
(d) X2Y3
Answer
B
Question. Where would you locate the element with electronic configuration 2, 8 in the modern periodic table?
(a) Group 8
(b) Group 2
(c) Group 18
(d) Group 10
Answer
C
Question. Which of the following statement(s) about the modern periodic table are incorrect?
(i) The elements in the modern periodic table are arranged on the basis of their decreasing atomic numbers.
(ii) The elements in the modern periodic table are arranged on the basis of their increasing atomic masses.
(iii) Isotopes are placed in adjoining group(s) in the periodic table.
(iv) The elements in the modern periodic table are arranged on the basis of their increasing atomic number.
(a) Only (i)
(b) (i), (ii) and (iii)
(c) (i), (ii) and (iv)
(d) Only (iv)
Answer
B
Question. Three elements B, Si and Ge are:
(a) Metals
(b) Non-metals
(c) Metalloids
(d) Metal, non-metal and metalloid respectively
Answer
C
Question. Which of the following elements will form an acidic oxide?
(a) An element with atomic number 7
(b) An element with atomic number 3
(c) An element with atomic number 12
(d) An element with atomic number 19
Answer
A
Question. An element X with atomic number 12 forms a compound with element Y with atomic number 17. The formula of the compound formed is:
(a) XY
(b) XY2
(c) X2Y
(d) X2Y3
Answer
B
Question. Elements P, Q, R and S have atomic numbers 11, 15, 17 and 18 respectively. Which of them are reactive non-metals?
(a) P and Q
(b) P and R
(c) Q and R
(d) R and S
Answer
C
Question. Which of the following set of elements is written in order of their increasing metallic character?
(a) Be, Mg, Ca
(b) Na, Li, K
(c) Mg, Al, Si
(d) C, O, N
Answer
A
Question. An element X makes an oxide with the formula X2O3. This element will be in the same group as:
(a) Na
(b) Mg
(c) Al
(d) Cl
Answer
C
Question. An element which is an essential constituent of all organic compounds belongs to:
(a) Group 1
(b) Group 14
(c) Group 15
(d) Group 16
Answer
B
Question. According to Mendeleev’s periodic law, the elements were arranged in the periodic table in the order of:
(a) Increasing atomic number
(b) Decreasing atomic number
(c) Increasing atomic masses
(d) Decreasing atomic masses
Answer
C
Question. On moving from left to right in a period in the periodic table, the size of the atom:
(a) increases
(b) decreases
(c) does not change appreciably
(d) first decreases and then increases
Answer
B
Question. Which of the following is the outermost shell for elements of period 2?
(a) K shell
(b) L shell
(c) M shell
(d) N shell
Answer
B
Question. Which of the following gives the correct increasing order of the atomic radii of O, F and N?
(a) O, F, N
(b) N, F, O
(c) O, N, F
(d) F, O, N
Answer
D
Question. The electronic configurations of three elements X, Y and Z are:
X : 2 Y : 2, 8, 7 Z : 2, 8, 2
Which of the following is correct regarding these elements?
(a) X is a metal
(b) Y is a metal
(c) Z is a non-metal
(d) Y is a non-metal and Z is a metal
Answer
D
Question. Which of the following elements would lose an electron easily?
(a) Mg
(b) Na
(c) K
(d) Ca
Answer
C
Question. Which of the following elements does not lose an electron easily?
(a) Na
(b) F
(c) Mg
(d) Al
Answer
B
Question. Which of the following are the characteristics of isotopes of an element?
(i) Isotopes of an element have the same atomic masses.
(ii) Isotopes of an element have the same atomic number.
(iii) Isotopes of an element show the same physical properties.
(iv) Isotopes of an element show the same chemical properties.
(a) (i), (iii) and (iv)
(b) (ii), (iii) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer
D
Question. Which of the following statements about the modern periodic table is correct?
(a) It has 18 horizontal rows known as periods.
(b) It has 7 vertical columns rows known as periods.
(c) It has 18 vertical columns known as groups.
(d) It has 7 horizontal rows known as groups.
Answer
C
Question. Arrange the following elements in the order of their increasing non-metallic character Li, O, C, Be and F.
(a) F < O < C < Be < Li
(b) Li < Be < C < O < F
(c) F < O < C < Be < Li
(d) F < O < Be < C < Li
Answer
B
Question. What type of oxide would Eka-aluminium form?
(a) EO3
(b) E3O2
(c) E2O3
(d) EO
Answer
C
Question. The element with atomic number 14 is hard and forms acidic oxide and a covalent halide.
To which of the following categories does the element belong?
(a) Metal
(b) Metalloid
(c) Non-metal
(d) Left-hand side element
Answer
B
Question. Which one of the following depicts the correct representation of atomic radius (r) of an atom? (Image 110)
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv)
Answer
B
Question. Which one of the following does not increase while moving down the group of the periodic table?
(a) Atomic radius
(b) Metallic character
(c) Valence
(d) Number of shells in an element
Answer
C
Assertion-Reason (A-R) Questions
For the following questions two statements are given- one labeled Assertion (A) and the other labeled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
(a) Both (A) and (R) are true and (R) is correct explanation of the assertion.
(b) Both (A) and (R) are true but (R) is not the correct explanation of the assertion.
(c) (A) is true but (R) is false.
(d) (A) is false but (R) is true.
Question. Assertion (A) : Chemical properties of the elements belonging to the same group are the same.
Reason (R) : Elements belonging to the same group possess the same number of valence electrons.
Answer : (a)
Question. Assertion (A) : In the Modern Periodic Table, the non-metals are found on the right side of the periodic table towards the top.
Reason (R) : The ability to gain electrons decreases as we move from left to right across a period.
Answer : (c)
Question. Assertion (A) : Calcium (Atomic No. 20) is more metallic than Potassium (Atomic No. 19).
Reason (R) : The tendency to lose electrons decreases as we move from left to right in a period.
Answer : (c)
Question. Assertion (A) : Newland’s law of Octaves worked well with lighter elements only.
Reason (R) : Newland assumed that only 56 elements existed in nature.
Answer : (b)
Question. Assertion (A) : Potassium has a bigger atomic radius than lithium.
Reason (R) : Atomic radius decreases along a period.
Answer : (b)
Question. Assertion (A) : A correct position could not be assigned to hydrogen in the Mendeleev’s periodic table.
Reason (R) : Mendeléev Periodic Law states that ‘the properties of elements are the periodic function of their atomic numbers’.
Answer : (c)
Question. Assertion (A) : The elements Sodium (Na), Magnesium (Mg), Aluminium (Al) belong to the same group in Modern Periodic Table.
Reason (R) : Atoms of different elements with the same number of occupied shells are placed in the same period.
Answer : (d)
Question. Assertion (A) : The atomic radii of the first 3 elements of the first group in Modern Periodic Table can be shown as K > Na > Li, where radius is in pm.
Reason (R) : The atomic size increases down a group.
Answer : (a)
Question. Assertion (A) : Nitrogen is a non-metal.
Reason (R) : Nitrogen has 5 valence electrons.
Answer : (a)

