Sample Paper Class 12 Chemistry
Get Class 12 Chemistry Sample Papers free pdf download which is based on the latest pattern of CBSE and NCERT. It involves every one of the points given in NCERT class 12 Chemistry book. You can easily download sample paper accounts class 12 is given below.
Download 12th Chemistry Question paper in PDF free of charge. It will help you to make your preparation better to score higher marks in exams. These Class 12 Chemistry Sample Papers PDFs are prepared by our expert teacher.
This 12th Chemistry Question Paper PDF assists you with revising the complete chapter in minutes. One of the best tips suggested by teachers is Solving the sample papers during exam time.
We bring here the latest collection of Sample Papers for Class 12 Chemistry prepared as per the latest examination pattern issued by CBSE. Students can refer to the latest paper below available with answers and also download the suggested guess papers in PDF format for free. Students should solve the papers in exam type environment at home and then compare their results with the answers provided below. Students should regularly solve questions given in DK Goel Class 12 book and also solve the papers given below
Sample Paper Class 12 Chemistry Term 2 Set A
Section A
1. Out of the following pairs, predict with reason which pair will allow greater conduction of electricity
(a) Silver wire at 30°C or silver wire at 60°C
(b) 0.1 M CH3COOH solution or 1M CH3COOH solution
(c) KCl solution at 20°C or KCl solution at 50°C
Answer. (a) Silver wire at 30°C because conductance of metals increase with decrease in temperature due to decrease in resistance.
(b) 0.1 M CH3COOH due to lower concentration, degree of ionisation increases.
(c) KCl at 50°C because mobility of ions increases with increase in temperature.
2. (a) Arrange the following in increasing order of pKa.
CH3CH2COOH, CH3COOH, C6H5COOH, C6H5CH2COOH
(b) Arrange the following in increasing order of reactivity towards NaHSO3
Acetone, Acetaldehyde, Acetophenone, Formaldehyde
Answer. (a) C6H5COOH < C6H5CH2COOH < CH3COOH < CH3CH2COOH
(b) Acetophenone < Acetone < Acetaldehyde < Formaldehyde
3. (a) Write IUPAC name of CH3 — CH = CH —COOH
(b) How will you distinguish between acetone and acetaldehyde?
Answer. (a) But-2-enoic acid
(b) Add Tollen’s reagent. Acetaldehyde will form silver mirror whereas acetone will not react
Section B
4. Complete the following

Answer. (a) ‘A’ is CH3CONH2, ‘B’ is CH3NH2
(b) ‘A’ is C6H5NH2, ‘B’ is C6H5N2 + Cl–
(c) ‘A’ is C6H5C ≡ N ‘B’ is C6H5COOH
OR
(a) How will you distinguish between the following pairs of compounds?
(i) Aniline and ethanamine (ii) Aniline and N-methyl aniline
(b) Arrange the following compounds in decreasing order of their boiling points. Butanol, Butanamine, Butane
Answer. (a) (i) Add NaNO2 + HCl. Cool it to 0 – 5°C. Add alkaline solution of phenol.
Aniline will form orange azo dye, ethanamine will not.
(ii) Add CHCl3 and KOH (alc). Aniline will form offensive smelling compound, phenyl isocyanide, N-Methyl aniline will not react.
(b) Butanol > Butanamine > Butane
5. (a) Write electronic configuration of iron ion in the following complex ion and predict its magnetic behaviour in
[Fe(H2O)6]2f (Fe = 26)
(b) Write IUPAC name of [CoCl2(en)2] NO3
(c) Predict the geometry of [Cu(NH3)4]2+
Answer. (a) t2g4,eg2, it has four unpaired electrons. [H2O is weak field ligand]
(b) Dichloridobis (Ethane-1, 2-diamine) cobalt (III) nitrate.
(c) It has square planar geometry due to dsp2 hybridisation.
OR
(a) Give the formula of potassium tetrahydroxidozincate II.
(b) Why [Ni(CN)4]2– is diamagnetic whereas [NiCl4]2– paramagnetic?
(c) [Ti(H2O)6]3+ is violet whereas (Ti(H2O)6)4+ is colourless. Why?
Answer. (a) K2[Zn(OH)4]
(b) [Ni(CN)4]2– does not have unpaired electrons whereas (NiCl4)2– has unpaired elections.
(c) [Ti(H2O)6]3+ has one unpaired electron. It can undergo d-d transition by absorbing energy from visible region and radiates purple colour whereas [Ti(H2O)6]4+ does not have unpaired electrons.
6. Give reasons for the following.
(a) Transition metals act as catalyst.
(b) It is difficult to obtain oxidation state greater than two for copper.
(c) CrO is basic but Cr2O3 is amphoteric.
Answer. (a) It is due to large surface area and ability to show variable oxidation state.
(b) It is due to high third ionisation energy.
(c) Oxidation state of Cr in Cr2O3 is +3 and CrO is +2. Higher the oxidation state, lesser will be ionic character. That is why CrO is basic but, Cr2O3 is amphoteric.
7. Observed and calculated values for standard electrode potentials of elements from Ti to Zn in the first series are depicted in the diagram.
Explain the following observations.
(a) The general trend towards less negative E° values across the series.
(b) The unique behaviour of copper.
(c) More negative E° values of Mn and Zn.
Answer. (a) It is due to increase in sum of first and second ionisation enthalpies.
(b) The high energy needed to convert Cu(s) to Cu2+(aq) is not balanced by hydration energy.
(c) Mn2+ (3d5) is more stable due to half filled d orbitals and Zn2+(3d10) is more stable due to completely filled d-orbitals.

OR
Give reason of the following.
(a) Transition metals form alloys.
(b) Zinc has lowest enthalpy of atomisation.
(c) Manganese shows higher oxidation state of +4 with fluorine but shows +7 with oxygen.
Answer. (a) It is due to similar atomic size, they can replace each other in metallic bond.
(b) It is due to weak metallic bonds due to larger size and absence of unpaired electrons.
(c) It is because ‘F’ cannot form double bond whereas oxygen can form double bonds.
8. (a) Why do true solution not show Tyndall effect?
(b) Lyophilic sols are more stable than lyophobic sols. Why?
(c) When KI is added to excess of AgNO3, positively charged AgI sol is formed. Why?
Answer. (a) The particles of true solutions are very small (<1 nm), do not scatter light, and hence, do not show Tyndall effect.
(b) In lyophilic sols, there is strong attraction between dispersed phase and dispersion medium as compared to lyophobic sols.
(c) It is due to adsorption of Ag+ due to excess of AgNO3 forming AgI/Ag+.
9. Arrange the following in increasing order of property specified.
(a) Aniline, ethanamine, N-ethyl ethanamine (solubility in water)
(b) Ethanoic acid, ethanamine, ethanol (boiling point)
(c) Methenamine, N, N-dimethyl methanamine, N-methyl methanamine (basic strength in aquous phase)
Answer. (a) Aniline < N-ethyl ethanamine < ethanamine
(b) Ethanamine < ethanol < ethanoic acid
(c) N, N-dimethyl methanamine < methanamine < N-methyl methanamine
OR
(a) Give a chemical test to distinguish between N-methyl ethanamine and N, N-dimethyl ethanamine.
(b) Write the reaction for the catalytic reduction of nitrobenzene followed by reaction of product formed with Bromine water.
(c) Out of butan-l-ol and butan-1-amine, which will be more soluble in water and why?
Answer. (a) Add Hinsberg reagent. N-methyl ethanamine will form compound insoluble in KOH while N, N – dimethyl ethanamine will not react.
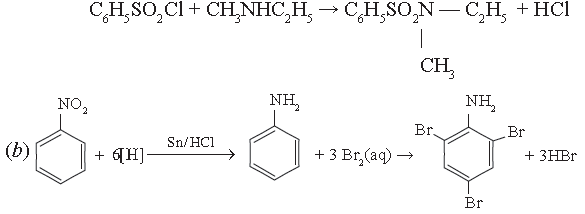
(c) Butan-1-ol will be more soluble in water because alcohols form stronger H – bonds than amines as oxygen is more electronegative than nitrogen.
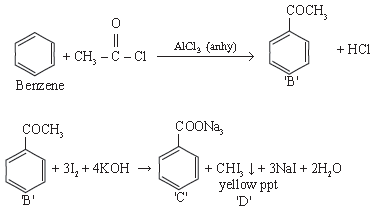
10. An organic compound ‘A’ C8H6 on treatment with dil H2SO4 containing HgSO4 gives a compound ‘B’. ‘B’ can be obtained by reaction of benzene with acetyl chloride in presence of AlCl3(only). ‘B’ on treatment with I2 / KOH gives ‘C’ and a yellow ppt on ‘D’. Identify A, B, C and D. Give the chemical reaction involved.
Answer.

11. Calculate the emf of cell for the following cell reaction at 298 K and also represent the cell E°cell = 2.204V, Mg(s) + Sn2+ (0.1M) → Mg2+ (0.01M) + Sn(s) [log 10 = 1] [log10–1 = – 1]
Answer. Mg(s) / Mg2+ (0.01 M) || Sn2+ (0.1M) / Sn(s)

Section C
12. Read the passage given below and answer the questions that follow.
Most literature data for the change in food quality based either on some chemical reaction, microbial growth, death or sensory value follow a zero order or first order kinetics. The integrated equations (1) and (2) for the reaction are.
Zero order, loss [A] = [A]0 – k0t
Gain [B] = [B]0 + k0t

(Source: T.P. Labzua, Department of Food Science and Nutrition, University of Minnesota St. Paul MN 55/08 page. 358)
(a) What is order of photo chemical reaction?
(b) If half life of reaction is independent of initial concentration, what is order of reaction?
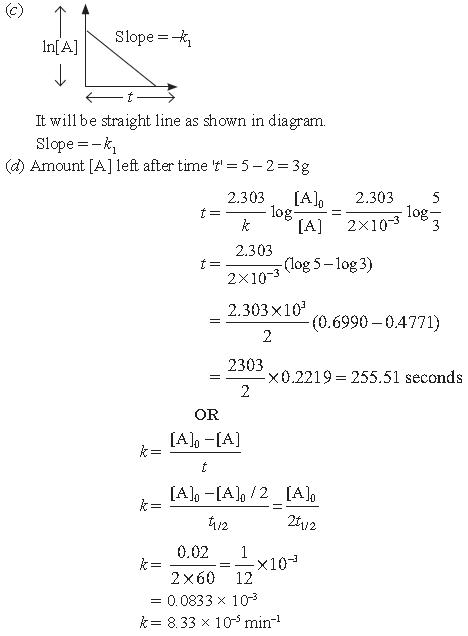
(c) If we plot a graph between ln[A] vs time, what will be nature of graph and slope? Draw the graph.
(d) A first order reaction has rate constant 2 × 10–3 s–1. How long will it take 5g of A change to 2g of B?
[log5 = 0.6990 log 3 = 0.4771 log2 = 0.3010]
OR
The time required to decompose ‘A’ to half of its initial amount is 60 minutes. If decomposition is zero order, calculate, the rate constant if initial concentration of ‘A’ is 0.02 M.
Answer. (a) Zero order
(b) First order