MCQs for Science Class 10 with Answers Chapter 4 Carbon and Its Compound
Students of class 10 Science should refer to MCQ Questions Class 10 Science Carbon and Its Compound with answers provided here which is an important chapter in Class 10 Science NCERT textbook. These MCQ for Class 10 Science with Answers have been prepared based on the latest CBSE and NCERT syllabus and examination guidelines for Class 10 Science. The following MCQs can help you to practice and get better marks in the upcoming class 10 Science examination
Chapter 4 Carbon and Its Compound MCQ with Answers Class 10 Science
MCQ Questions Class 10 Science Carbon and Its Compound provided below have been prepared by expert teachers of grade 10. These objective questions with solutions are expected to come in the upcoming Standard 10 examinations. Learn the below provided MCQ questions to get better marks in examinations.
Question. Which of the following is not a straight chain hydrocarbon?
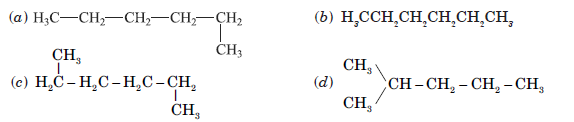
Answer
D
Question: Which of the following options is false about a soap?
(a) The soap solution in water is neutral and can be used to wash all kinds of fabrics.
(b) Soap forms lather only in soft water.
(c) Soap is a metallic salt of higher fatty acids.
(d) Soap cannot be used in slightly acidic medium.
Answer
A
Question: Structural formula of benzene is:

Answer
C
Question: What does isomerism explain?
(a) A difference in molecular formulae.
(b) A difference in molecular weights.
(c) A difference in chemical properties and structural formulae.
(d) A difference in molecular composition.
Answer
C
Question: Buckminister fullerene is an allotropic form of
(a) phosphorus
(b) sulphur
(c) carbon
(d) tin
Answer
C
Question: The correct name of the given compound is:
(a) 2, 3-diethyl heptane
(b) 5-ethyl-6-methyl octane
(c) 4-ethyl-3-methyl octane
(d) 3-methyl-4-ethyl octane
Answer
C
Question: Which is a general formula of alkenes?
(a) CnH2n+2
(b) CnH2n
(c) CnH2n–2
(d) None of these
Answer
B
Question: The functional group represent alcohol is –
(a) – OH
(b) – CHO
(c) – COOH
(d) > C = O
Answer
A
Question: Which of the following is the purest form of carbon?
(a) charcoal
(b) coal
(c) diamond
(d) graphite
Answer
C
Question:The number of 4° carbon atoms in 2,2,4,4-tetramethyl pentane is –
(a) 1
(b) 2
(c) 3
(d) 4
Answer
B
Question: Organic compounds will always contain –
(a) carbon
(b) hydrogen
(c) nitrogen
(d) sulphur
Answer
A
Question: When methane is burnt in an excess of air, the products of combustion are –
(a) C and H2O
(b) CO and H2O
(c) CO2 and H2
(d) CO2 and H2O
Answer
D
Question: Which of the following gases is called ‘marsh gas’?
(a) H2
(b) CH4
(c) C2H4
(d) C2H2
Answer
B
Question: The final product of chlorination of methane in the sun light is –
(a) CH3Cl
(b) CH2Cl2
(c) CHCl3
(d) CCl4
Answer
D
Question:The number of oxygen molecules used in the combustion of 1 molecule of ethanol is –
(a) 1
(b) 2
(c) 3
(d) 4
Answer
C
Question: Methane, ethane and propane are said to form a homologous series because all are –
(a) hydrocarbons
(b) saturated compounds
(c) aliphatic compounds
(d) differ from each other by a CH2 group
Answer
D
Question: General formula of alkyne is –
(a) CnH2n+2
(b) CnH2n
(c) CnH2n–2
(d) CnHn
Answer
C
Question: When vanaspati oil reacts with hydrogen then it is converted into vanaspati ghee. In this process catalystused is :
(a) Fe
(b) Mo
(c) V
(d) Ni
Answer
D
Question: Observe the following pairs of organic compounds :
(I) C4H9OH and C5H11OH
(II) C7H15OH and C5H11OH
(III) C6H13OH and C3H7OH
Which of these pair is a homologous series according to increasing order of carbon atom?
(a) (III) only
(b) (II) only
(c) (I) only
(d) All of these
Answer
C
Question: Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of :
(a) addition reaction
(b) substitution reaction
(c) displacement reaction
(d) oxidation reaction
Answer
A
Question: Carbon exists in the atmosphere in the form of :
(a) carbon monoxide only.
(b) carbon monoxide in traces, and carbon dioxide.
(c) carbon dioxide only.
(d) coal
Answer
B
Question: Chlorine reacts with saturated hydrocarbons at room temperature in the
(a) absence of sunlight
(b) presence of sunlight
(c) presence of water
(d) presence of hydrochloric acid
Answer
B
Question: Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g. hydrogen. After the formation of four bonds, carbon
attains the electronic configuration of:
(a) helium
(b) neon
(c) argon
(d) krypton
Answer
B
Question: Which of the following does not belong to the same homologous series?
(a) CH4
(b) C2H6
(c) C3H8
(d) C4H8
Answer
D
Question: The enzyme involved in the oxidation of ethanol to form vinegar is –
(a) zymase
(b) oxidase
(c) acetobacter
(d) invertase
Answer
C
Question: When ethanoic acid is heated with NaHCO3 the gas evolved is –
(a) H2
(b) CO2
(c) CH4
(d) CO
Answer
B
Question. A soap molecule has a
(a) hydrophobic head and hydrophobic tail
(b) hydrophobic head and hydrophilic tail
(c) hydrophilic head and hydrophilic tail
(d) hydrophilic head and hydrophobic tail
Answer
D
Question. Assertion: Soaps are 100% biodegradable but do not work well with hard water.
Reason: Some detergents are not bio-degradable but work well with hard water.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
(e) Both A and R are false.
Answer
B
Question. Why does carbon form compounds mainly by covalent bonding?
(a) There are four electrons in the outermost shell of carbon.
(b) It requires large amount of energy to form C4+ or C4–
(c) It shares its valence electrons to complete its octet.
(d) All the above
Answer
D
Question. Alcohols can be produced by the hydration of
(a) Alkenes
(b) alkynes
(c) alkanes
(d) acids
Answer
A
Question. The number of covalent bonds in C4H10 is
(a) 10
(b) 8
(c) 13
(d) 12
Answer
C
Question. Which of the following statements about graphite and diamond is true?
(a) They have the same crystal structure
(b) They have the same degree of hardness
(c) They have the same electrical conductivity
(d) They can undergo the same chemical reactions
Answer
D
Question. Ethanol reacts with Na metal to form
(a) CH3ONa + H2
(b) C2H5ONa + H2
(c) CH3COONa + H2
(d) CH3C00H + H2O
Answer
B
Question. Butanone is a four carbon compound with the functional group
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Answer
C
Question. Which of the following will undergo addition reactions?
(a) CH4
(b) C3H8
(C) C2H6
(d) C2H4
Answer
D
Question. The odour of acetic acid resembles that of
(a) Rose
(b) Burning Plastic
(c) Vinegar
(d) Kerosene
Answer
C
Question. The number of C-H bonds in ethane C2H6 molecule are
(a) 4
(b) 6
(c) 8
(d) 10
Answer
B
Question. Ethane and ethene can be distinguished by
(a) Br2(l)
(b) Br2 (aq) water
(c) Cl2
(d) I2
Answer
B
Question. Which amongst the following will conduct electricity?
(a) C6H12O6
(b) KCl(s)
(c) C2H5OH
(d) NaCl (aq)
Answer
D
Question. Carbon exists in the atmosphere in the form of
(a) carbon monoxide only
(b) carbon monoxide in traces and carbon dioxide
(c) carbon dioxide only
(d) coal
Answer
B
Question. Which of the following is ethanol?
(a) CH3CHO
(b) CH3COOH
(c) CH3CH2
(d) CH3COOCH3
Answer
C
Question. Which of the following contains covalent bond?
(a) MgCl2
(b) CaF2
(c) Al2O3
(d) HCl
Answer
D
Question.The by product in soap industry is
(a) Isoprene
(b) Ethylene glycol
(c) glycerol
(d) butane
Answer
C
Question. When ethanoic acid is treated with NaHCO the gas evolved is
(a) H2
(b) CO2
(c) CH4
(d) CO
Answer
B
Question. Diamond is not a good conductor of electricity because
(a) It is very hard
(b) Its structure is very compact
(c) It is not soluble in water
(d) It has no free electrons to conduct electric current.
Answer
D
Question. The first compound to be prepared in the laboratory was
(a) Methane
(b) Ethyl alcohol
(c) acetic acid
(d) Urea
Answer
D
Question. While cooking, if the bottom of the vessels is getting blackened on the outside, it means that
(a) the fuel is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the is burning completely.
Answer
B
Question. Ethanol on complete oxidation gives
(a) acetic acid/ethanoic acid
(b) CO2 and water
(c) ethanal
(d) acetone/ethanone
Answer
B
Question. Addition reactions are undergone by
(a) saturated hydrocarbons (alkanes)
(b) only alkenes
(c) only alkynes
(d) both alkenes and alkynes
Answer
D
Question. The difference in the formula and molecular masses of CH3OH and C2H5OH is
(a) CH3 and 16u
(b) CH2 and 14u
(c) CH4 and 18u
(d) CH3 and 16u
Answer
B
Question. The self linkage property (catenation) is maximum in
(a) carbon
(b) silicon
(c) sulphur
(d) phosphorus
Answer
A
Question. – CHO represents the functional group
(a) esters
(b) carboxylic acid
(c) alcohols
(d) aldehydes
Answer
D
Question. Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of
(a) Addition reaction
(b) Substitution reaction
(c) Displacement reaction
(d) Oxidation reaction
Answer
A
Question: C3H8 belongs to the homologous series of
(a) Alkynes
(b) Alkenes
(c) Alkanes
(d) Cyclo alkanes
Answer
C
Question: The number of isomers of pentane is
(a) 2
(b) 3
(c) 4
(d) 5
Answer
B
Question: Which of the following statements are correct for carbon compounds?
(i) Most carbon compounds are good conductors of electricity.
(ii) Most carbon compounds are poor conductors of electricity.
(iii) Force of attraction between molecules of carbon compounds is not very strong.
(iv) Force of attraction between molecules of carbon compounds is very strong.
(a) (ii) and (iv)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (i) and (iii)
Answer
B
Question: When ethanoic acid is treated with NaHCO^ the gas evolved is
(a) H2
(b) CO2
(c) CH4
(d) CO
Answer
B
Question: Ethanol on complete oxidation gives
(a) acetic acid/ethanoic acid
(b) CO2 and water
(c) ethanal
(d) acetone/ethanone
Answer
B
Question: Which of the following will undergo addition reactions?
(a) CH4
(b) C3H8
(C) C2H6
(d) C2H4
Answer
D
Question: Name the functional group present in CH3COCH3.
(a) Alcohol
(b) Carboxylic acid
(c) Ketone
(d) Aldehyde
Answer
C
Question: Which of the following will give a pleasant smell of ester when heated with ethanol and a small quantity of sulphuric acid?
(a) CH3COOH
(b) CH3CH2OH
(c) CH3OH
(d) CH3CHO
Answer
A
Question. IUPAC name of first member of homologous series of ketones is
(a) Ethanone
(b) methanone
(c) Propanone
(d) Butanone
Answer
C
A ssertion-Reason Type Questions
For question numbers 1 and 2 two statements are given-one labeled as Assertion (A) and the other labeled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
(a) Both ‘A’ and ‘R’ are true and ‘R’ is correct explanation of the assertion.
(b) Both ‘A’ and ‘R’ are true but ‘R’ is not correct explanation of the assertion.
(c) ‘A’ is true but ‘R’ is false.
(d) ‘A’ is false but ‘R’ is true.
Question. Assertion: C6H12, C2H4, C4H8 are alkenes and have double bond.
Reason: C3H4 and C5H8 are alkynes and have triple bond.
Answer
B
Question. Assertion: Following are the members of a homologous series:
CH3OH, CH3CH2OH,CH3CH2CH2OH
Reason: A series of compounds with same functional group but differing by – CH2– unit is called a homologous series.
Answer
A

We hope the above multiple choice questions for Class 10 Science for Chapter 4 Carbon and Its Compound provided above with answers based on the latest syllabus and examination guidelines issued by CBSE, NCERT and KVS are really useful for you. Carbon and Its Compound is an important chapter in Class 10 as it provides very strong understanding about this topic. Students should go through the answers provided for the MCQs after they have themselves solved the questions. All MCQs have been provided with four options for the students to solve. These questions are really useful for benefit of class 10 students. Please go through these and let us know if you have any feedback in the comments section.
