Exam Question for Class 10 Chemistry Chapter 7 The p – Block Elements
Very Short Answer Type Questions
Question. Fluorine exhibits only –1 oxidation state whereas other halogens exhibit +1, +3, +5 and +7 oxidation states also. Why is it so?
Answer. It is because fluorine is the most electronegative element and it does not have d-orbitals.
Question. Though nitrogen exhibits +5 oxidation state, it does not form pentahalide. Why?
Answer. Due to absence of d-subshell in N atom.
Question. Write the formula of the compound of phosphorus which is obtained when conc. HNO3 oxidises P4.
Answer.

Question. Write the formula of the compound of sulphur which is obtained when conc. HNO3 oxidises S8.
Answer. S8 + 48HNO3 → 8H2SO4 + 48NO2 + 16H2O
Question. Why is red phosphorus less reactive than white phosphorus?
Answer. Because white phosphorus has angular strain in its P4 molecules where the angle is only 60°.
Question. Why does NO2 dimerise?
Answer. NO2 contains 7 + 2 × 8 i.e. 23 odd electrons. In the valence shell N has seven electrons and hence less stable. To acquire stability it dimerizes to form N2O4

Question.What is the oxidation number of phosphorus in H3PO2 molecule?
Answer. H3PO2
3 + x – 4 = 0 or x – 1 = 0 ∴ x = + 1
Thus oxidation number of P in H3PO2 = +1.
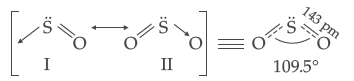
Question. Draw the structure of O3 molecule.
Answer. Structure of Ozone (O3) : Angular structure

Question. Nitrogen is relatively inert as compared to phosphorus. Why?
Answer. Because P – P single bond is much weaker than N≡ N triple bond and the bond length of nitrogen is small and bond dissociation energy is very large which makes it inert and unreactive and thus phosphorus becomes more reactive.
Question. Noble gases have low boiling points. Why?
Answer. Noble gases being monoatomic have no interatomic forces except weak dispersion force and therefore, they are liquified at very low temperature. Hence, they have low boiling point.
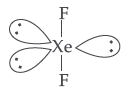
Question. Draw the structure of XeF2 molecule.
Answer. XeF2 :

Question. Which one of PCl4+ and PCl4− is not likely to exist and why?
Answer. PCl4− , because P has 10 electrons which cannot be accommodated in sp3 hybrid orbitals.
Question. Why is Bi(v) a stronger oxidant than Sb(v)?
Answer. The stability of +5 oxidation state decreases and that of +3 state increases due to inert pair effect down the group therefore Bi(v) accepts two electrons and gets reduced to Bi (v).
Bi5+ + 2e– → Bi3+
Question. What is the basicity of H3PO2 acid and why?
Answer. H3PO2 has one replaceable H atom so it is monobasic.
Question. Though nitrogen exhibits +5 oxidation state, it does not form pentahalide. Why?
Answer. Due to non-availability of d-orbitals in its valence electronic configuration nitrogen does not form pentahalide.
Short Answer Type Questions
Question. (a) Which form of sulphur shows paramagnetic behaviour and why ?
(b) Fluorine exhibits only – 1 oxidation state whereas other halogens exhibit +1, +3, +5 or +7 oxidation states also. Explain as to why.
Answer. (a) In vapour state sulphur partly exists as S2 molecule which has two unpaired electrons in the antibonding π* orbitals like O2 and hence exhibits paramagnetism.
(b) It is because fluorine is the most electronegative element and it does not have d-orbitals.
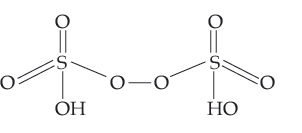
Question. Draw the structures of the following:
(a) H2S2O8 (b) ClF3
Answer. (a) Structure of H2S2O8 :

Question. (a) What is the covalence of nitrogen in N2O5?
(b) BiH3 is a stronger reducing agent than SbH3, why?
Answer. (a) The covalency of nitrogen in N2O5 is 4 because each nitrogen atom has four shared pairs of electrons.
(b) BiH3 : Because it is a stronger reducing agent as its tendency to liberate H is maximum.
Question. Account for the following :
(i) Two S-O bond lengths in SO2 are equal.
(ii) Fluorine shows only -1 oxidation state in its compounds.
Answer. (i) Due to resonance in SO2 the double bond (π) electrons are distributed equally in both resonating structures as a result of which the bond length of two S-O becomes equal
(ii) Because it is most electronegative element and does not have d-orbitals for octet expansion, therefore it shows only a negative oxidation state of –1.
Question. Account for the following:
(i) Bond angle is NH4 + is higher than that in NH3.
(ii) ICl is more reactive than I2.
Answer. (i) Because in NH4 + ion there is no lone pair of electrons which is present in NH3 due to which lone pair-bond pair repulsion occurs and bond angle decreases from 109°28’ to
107.3°.
(ii) Because I-Cl bond is weaker than I-I bond as a result of which ICl breaks easily to form halogen atoms which readily bring about the reaction, hence more reactive.
Question. Draw the structures of the following:
(i) H4P2O7 (ii) XeOF4
Answer. (i) H4P2O7

Short Answer Type Questions-II
Question. Give reasons for the following :
(i) Though nitrogen exhibits +5 oxidation state, it does not form pentahalide.
(ii) Electron gain enthalpy with negative sign of fluorine is less than that of chlorine.
(iii) The two oxygen-oxygen bond lengths in ozone molecule are identical.
Answer. (i) Due to absence of empty d-orbitals, N2 does not form pentahalide.
(ii) Because of small size of flourine atom and strong electron-electron repulsions in its compact 2p orbitals.
(iii) Due to resonance the two oxygen atoms have partial double bond character and thus have same bond length i.e. 128 pm

Question. Give reasons for the following :
(i) (CH3)3 P = O exists but (CH3)3 N = O does not.
(ii) Oxygen has less electron gain enthalpy with negative sign than sulphur.
(iii) H3PO2 is a stronger reducing agent than H3PO3.
Answer. (i) (CH3)3P = 0 exists due to presence of empty d-orbitals and thus can expand its covalency upto 6 but (CH3)3 N = O cannot expand its covalency due to absence of d-orbitals.
(ii) The least negative electron gains enthalpy of oxygen is due to small size and more interelectronic repulsion with coming electron.
(iii) H3PO2 contains two P-H bonds while H3PO3 contains only one P-H bond therefore H3PO2 is a stronger reducing agent.
Question. Give reasons:
(i) Thermal stability decreases from H2O to H2Te.
(ii) Fluoride ion has higher hydration enthalpy than chloride ion.
(iii) Nitrogen does not form pentahalide.
Answer. (i) Thermal stability decreases from H2O to H2Te due to weakening of bond between hydrogen and the atom from O to Te as size is increasing down the group.
(ii) Fluoride ion has higher hydration enthalpy than chloride ion due to stronger attractions of smaller in size fluoride ion.
(iii) Nitrogen does not contain ‘d’ orbitals.
Question. Explain the following situations :
(i) In the structure of HNO3 molecule, the
N– O bond (121 pm) is shorter than N –OH bond (140 pm).
(ii) SF4 is easily hydrolysed whereas SF6 is not easily hydrolysed.
(iii) XeF2 has a straight linear structure and not a bent angular structure.
Answer.

(i) In the structure the bond length of N – O is shorter due to formation of coordinate bond and double bond while in N– OH the bond is single covalent due to
which its bond length is greater than other N– O bond.
(ii) In SF4, due to less steric hindrance by four F atoms, H2O molecules can attack easily while in SF6 the S atom is completely protected by six F atoms and does not allow H2O molecules to attack the S atom.
(iii) In XeF2 there are 2 bond pairs and 3 lone pairs and thus show sp3d hybridization. It has linear geometry
Question. Give reasons for the following:
(a) Red phosphorus is less reactive than white phosphorus.
(b) Electron gain enthalpies of halogens are largely negative.
(c) N2O5 is more acidic than N2O3.
Answer. (a) Red phosphorus is less reactive than white phosphorus because white phosphorus possess angle strain where long angles are only 60º making it more reactive. Also, red phosphorus being polymeric is less reactive than white phosphorus which has discrete tetrahedral structure.
(b) Electron gain enthalpies of halogens are
largely negative due to high effective nuclear charge and smaller size among period. They readily accept an electron to attain noble gas configuration.
(c) N2O5 is more acidic than N2O3 because higher the oxidation state, higher will be acidic character. N2O5 has +5 oxidation state and N2O3 has +3 oxidation state.
Question. Draw the structures of white phosphorus and red phosphorus. Which one of these two types of phosphorus is more reactive and why?
Answer.

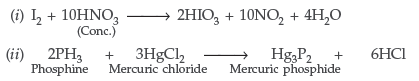
Question. Complete the following chemical reaction equations :
(i) I2 + HNO3 → (ii) HgCl2 + PH3 → (All India)
(Conc.)
Answer.
Question. State reasons for each of the following :
(i) The N–O bond in NO2– is shorter than the N–O bond in NO3– .
(ii) SF6 is kinetically an inert substance.
Answer. (i) The resonating structure of NO2– and NO3– show that in NO2– two bonds are sharing a double bond while in NO3–, 3 bonds are sharing a double bond. That’s why NO2– has shorter bond than that of NO3– .
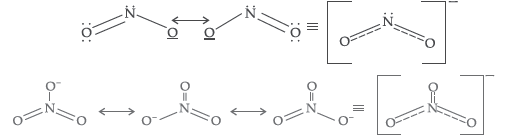
(ii) Because SF6 is showing steric hindrance due to 6 (six) fluorine atoms which make it unable to react further with any other atom.
Question. State reasons for each of the following :
(i) All the P-Cl bonds in PCl5 molecule are not equivalent.
(ii) Sulphur has greater tendency for catenation than oxygen.
Answer. (i) The PCl5 molecule has sp3d hybridization and trigonal bipyramidal geometry.
Therefore it has 3 equatorial P – Cl bonds and two axial P – Cl bonds. Since two axial P – Cl bonds are repelled by 3 bond pairs while 3 equatorial bonds are repelled by two bond pairs, so axial bonds are longer than equatorial bonds.

Question. Complete the following chemical equations :
(i) PCl5 Heat →
(ii) NaHCO3 + HCl ⎯⎯→
Answer.

Question. Complete the following chemical equations :
(i) SO2 + MnO4– + H2O →
(ii) F2 (g) + H2O (l) →
Answer.
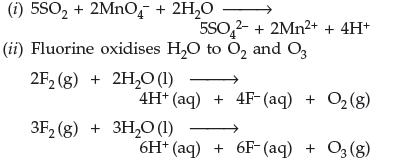
Question. Explain the following giving an appropriate reason in each case.
(i) O2 and F2 both stabilize higher oxidation states of metals but O2 exceeds F2 in doing so.
(ii) Structures of Xenon fluorides cannot be explained by Valence Bond approach.
Answer. (i) This is due to the ability of oxygen to form multiple bonds to metals.
(ii) This is because the energy required for the promotion of electrons in Xenon is very high.Energy factor does not favour VB approach.
Question. Explain the following :
(a) NO2 readily forms a dimer.
(b) BiCl3 is more stable than BiCl5.
Answer.
(b) BiCl3 is more stable than BiCl5 due to inert pair effect because as we move down the group, the stability of +3 oxidation state increases and of +5 decreases.
Question. Draw the structures of the following molecules :
(i) XeF4 (ii) BrF3
Answer.

Question. Explain the following :
(a) Xenon does not form such fluorides as XeF3 and XeF5.
(b) Out of noble gases, only Xenon is known to form real chemical compounds.
Answer. (a) By impairing of one paired orbital, two singly occupied orbitals come into existence.Thus, either two or four or six singly occupied orbitals can be formed instead of one, three or five singly occupied orbitals. Hence XeF, XeF3 or XeF5 are not formed.
(b) Xe atom has a large size and lower ionisation potential and hence the force of nucleus over the electrons is weak and hence very small energy can excite the electrons and hence it is easier for Xenon to form compounds than other noble gases.
Question. Complete the following equations :
(i) P4 + H2O →
(ii) XeF4 + O2F2 →
Answer.

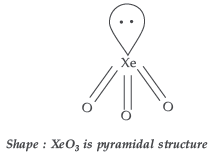
Question. How is XeO3 obtained? Write the related chemical equations. Draw the structure of XeO3.
Answer. Hydrolysis of XeF4 and XeF6 with water gives XeO3
6XeF4 + 12H2O → 4Xe + 2XeO3 + 24HF + 3O2 XeF6 + 3H2O → XeO3 + 6HF
Question. What happens when:
(i) SO2 gas is passed through an aqueous solution of Fe3+ salt?
(ii) XeF4 reacts with SbF5?
Answer. (i) In this sulphur dioxide acts as a reducing agent and reduces Fe3+ to Fe2+.
2Fe3+ + SO2 + 2H2O ⎯⎯→ 2Fe2+ + SO42– + 4H+
(ii) XeF4 + SbF5 ⎯⎯→ [XeF3]+ [SbF6]–.
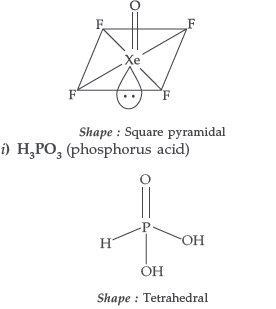
Question. Draw the structures of the following molecules:
(i) XeOF4 (ii) H3PO3
Answer.
Question. How are interhalogen compounds formed? What general compositions can be assigned to them?
Answer. Interhalogen compounds : Halogens react with each other to form a number of compounds called interhalogen compounds, whose general formula is XX′n.
where X = less electronegative atom (have larger size)
X′ = more electronegative atom (have smaller size)
n = no. of more electronegative atoms
They are of four types :
XX′ = ClF, BrF, IF, BrCl, ICl, IBr
XX′3 = ClF3, BrF3, IF3, ICl3
XX′5 = ClF5, BrF5, IF5
XX′7 = IF7
Naming : The halogen with positive oxidation state named first and with negative oxidation state named after first with suffix ‘ide’.
Example : BrCl3 → Bromine trichloride
IF7 → Iodine heptafluoride
Preparation of Interhalogen Compounds :
By direct combination : Example :

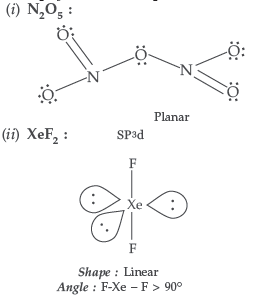
Question. Draw the structures of the following molecules :
(i) N2O5 (ii) XeF2
Answer.
Question. “Orthophosphoric acid (H3PO4) is not a reducing agent whereas hypophosphorus acid (H3PO2) is a strong reducing agent.” Explain and justify the above statement with the help of a suitable example.
Answer. Orthophosphoric acid (H3PO4) is not a reducing agent because it doesn’t contain any P-H bond whereas hypophosphorus acid (H3PO2) is a strong reducing agent as it contains two P-H bonds. H3PO2 can reduce silver nitrate (AgNO3) into metallic silver which H3PO4 can not.
4AgNO3 + H3PO2 + 2H2O → 4Ag ↓ + H3PO4 + 4HNO3
Question. Complete the following chemical equations :
(i) Ca3P2 + H2O →
(ii) Cu + H2SO4 (conc.) →
Answer. (i) Ca3P2 + 6H2O → 2PH3 + 3Ca(OH)2
(ii) Cu + 2H2SO4 (conc.) → CuSO4 + 2H2O + SO2
Question. What happens when H3PO3 is heated?
Answer.

Question. Complete the following chemical reaction equations :
(i) XeF2 (s) + H2O (l) → (ii) PH3 + HgCl2 →
Answer.

Question. Draw the structures of the following:
(i) H2S2O7 (ii) XeF6
Answer.

Question. Arrange the following in the order of property indicated against each set :
(i) HF, HCl, HBr, HI – increasing bond dissociation enthalpy.
(ii) H2O, H2S, H2Se, H2Te – increasing acidic character.
Answer. (i) HI < HBr < HCl < HF
(ii) H2O < H2S < H2Se < H2Te
Question. (a) Draw the structures of the following molecules :
(i) XeOF4 (ii) H2SO4
(b) Write the structural difference between white phosphorus and red phosphorus.
Answer.

(b) White phosphorus exists as discrete P4 units with SP3 hybridized phosphorus atom, arranged tetrahedrally but in red phosphorus all P4 tetrahedral units are linked with each other to form polymeric structure.
Question.Explain the following observations :
(i) Fluorine does not exhibit any positive oxidation state.
(ii) The majority of known noble gas compounds are those of Xenon.
(iii) Phosphorus is much more reactive than nitrogen.
Answer. (i) Because it is most electronegative element and does not have d-orbitals for octet expansion, therefore it shows only a negative oxidation state of – 1.
(ii) Because xenon has least ionization energy among noble gases and hence it readily forms chemical compounds particularly with oxygen and fluorine.
(iii) Because P – P single bond is much weaker than N ≡ N triple bond and the bond length of nitrogen is small and bond dissociation energy is very large which makes it inert and unreactive and thus phosphorus becomes more reactive.
Question. How would you account for the following :
(i) NCl3 is an endothermic compound while NF3 is an exothermic one.
(ii) XeF2 is a linear molecule without a bend.
(iii) The electron gain enthalpy with negative sign for fluorine is less than that for chlorine, still fluorine is a stronger oxidising agent than chlorine.
Answer. (i) F is more electronegative than Cl.
The difference in the electronegativity between N and F is much more than the difference between electronegativity of N and Cl. So there is need of much more energy to break the N – F bond.
(iii) Because of small size of flourine atom and strong electron–electron repulsions in its compact 2p orbitals.
Question. How would you account for the following :
(i) H2S is more acidic than H2O.
(ii) The N – O bond in NO2– is shorter than theN– O bond in NO3– .
(iii) Both O2 and F2 stabilize high oxidation states but the ability of oxygen to stabilize the higher oxidation state exceeds that of fluorine.
Answer. (i) Since the size of sulphur is more than oxygen, S – H bond length increases and hence bond dissociation energy of S – H is less than O – H. Therefore S – H easily loses H+ and thus is more acidic than H2O.
(iii) Oxygen stabilizes the highest oxidation state even more than fluorine.
Example : Highest fluoride of Mn is MnF4 whereas highest oxide is Mn2O7. It is due to ability of oxygen to form multiple bonds with the metal atoms.
Question. How would you account for the following :
(i) NF3 is an exothermic compound but NCl3 is not.
(ii) The acidic strength of compounds increases in the order :
PH3 < H2S < HCl.
(iii) SF6 is kinetically inert.
Answer.
(ii) As the electronegativity increases in the same period from left to right so their electronegativity are in the increasing order,P < S < Cl.
In the same way the acid strength is also in the increasing order i.e. PH3 < H2S < HCl.
(iii) Because SF6 is showing steric hindrance due to 6 (six) fluorine atoms which make it unable to react further with any other atom.
Question. Account for the following :
(i) Bi(V) is a stronger oxidizing agent than Sb(V).
(ii) N – N single bond is weaker than P – P single bond.
(iii) Noble gases have very low boiling points.
Answer. (i) Bi(V) is a stronger oxidizing agent than Sb(V) due to inert pair effect as the stability of lower oxidation state (+3) increases down the group.
(ii) Due to smaller size of Nitrogen, their lone pairs repel the bond pair of N – N bond while P – P due to bigger size does not show more repulsion.
(iii) Due to presence of weak Van der waal forces of attraction, noble gases have very low boiling point.
Question. Give reasons for the following:
(i) Where R is an alkyl group, R3P = O exists but R3N = O does not.
(ii) PbCl4 is more covalent than PbCl2.
(iii) At room temperature, N2 is much less reactive.
Answer. (i) Due to presence of d-orbitals in P, it can form pπ-dπ bonds and can extend its covalency beyond 4 while N cannot do so due to absence of d-orbitals.
(ii) According to Fajan’s rule, highly charged Pb4+ can polarise the anion i.e., Cl– more effectively than Pb2+ and hence PbCl4 becomes more covalent than PbCl2.
(iii) Due to presence of triple bonds between 2 N atoms, their bond length decreases and hence bond dissociation energy increases which makes N2 lesser reactive. While in phosphorus due to presence of single bond, more bond length, bond dissociation energy is low, hence very reactive.
Question. How would you account for the following :
(i) The electron gain enthalpy with negative sign is less for oxygen than that for sulphur.
(ii) Phosphorus shows greater tendency for catenation than nitrogen.
(iii) Fluorine never acts as the central atom in polyatomic interhalogen compounds.
Answer. (i) The least negative electron gain enthalpy of oxygen is due to small size and more interelectronic repulsion with coming electron.
(ii) The bond strength of P–P is more than N–N,therefore phosphorus shows more tendency for catenation than nitrogen.
(iii) Because F being smaller, it cannot accomodate larger sized other halogen atoms around it. Due to the absence of d-orbitals, F does not show positive oxidation state of +3, +5, +7 needed for the formation of polyatomic interhalogen compounds.
Question. (a) Name the gas evolved on heating ammonium nitrate. Write the chemical reaction.
(b) Write two uses of ammonium nitrate.
Answer. (a) The gas evolved on heating is Nitrous oxide
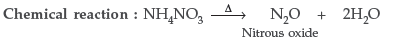
(b) Uses of NH4NO3
(i) It is used in fertilizers. (ii) It is used in explosives.
Question. Account for the following :
(i) NH3 is a stronger base than PH3.
(ii) Sulphur has a greater tendency for catenation than oxygen.
(iii) Bond dissociation energy of F2 is less than that of Cl2.
Answer. (i) Since both P and N contain lone pairs of electrons but due to small size and high electronegativity of Nitrogen in NH3 , the electron density is much higher than PH3 , therefore it can easily donate electrons and acts as strong Lewis base than PH3 .
(ii) The greater catenation tendency of sulphur is due to two reasons :
(a) The lone pair of electrons feels more repulsion in O – O bond than S – S bond due to its small size and thus S – S forms strong bond.
(b) As the size of atom increases down the group from O – PO, the strength of bond increases and therefore catenation tendency also increases.
(iii) Due to smaller size of F than Cl as a result of which electron-electron repulsions between the lone pairs of electrons are very large than that of Cl, hence bond dissociation energy of F2 is less than that of Cl2.
Question. Account for the following :
(i) NF3 is an exothermic compound but NCl3 is an endothermic compound.
(ii) HF is not stored in glass bottles but is kept in wax-coated bottles.
(iii) Bleaching of flowers by Cl2 is permanent while that of SO2 is temporary.
Answer.
(ii) HF is highly corrosive and etches glass hence it is kept in wax-coated bottles.
(iii) Chlorine bleaches the material by oxidation hence it is permanent while SO2 bleaches the material by reduction and as the material is exposed to air, it gets oxidised and the colour is restored, hence it is temporary.
Question. Give reasons:
(i) SO2 is reducing while TeO2 is an oxidizing agent.
(ii) Nitrogen does not form pentahalide.
(iii) ICl is more reactive than I2.
Answer. (i) SO2 is reducing while TeO2 is an oxidising agent because sulphur can expand its covalency upto +6 from +4 due to presence of empty d-orbital but as we move down the group the stability of +6 oxidation state decreases and of +4 oxidation state increases due to inert pair effect. Hence SO2 acts as reducing agent while TeO2 acts as an oxidising agent.
(ii) Due to absence of empty d-orbitals, N2 does not form pentahalides.
(iii) Because ICl bond is weaker than I – I bond as a result of which ICl breaks easily to form halogen atoms which readily bring about the reaction, hence more reactive.
Question. (a) With the help of chemical equations explain the principle of contact process in brief for the manufacture of sulphuric acid by contact process.
(b) Bismuth is a strong oxidizing agent in the pentavalent state. Explain.
Answer.
(b) The stability of +5 oxidation state decreases and that of +3 state increases due to inert pair effect down the group therefore Bi (V) accepts two electrons and gets reduced to Bi (III).
Bi5+ + 2e– → Bi3+
So, Bi(V) is more stronger oxidising agent.
Question. Account for the following :
(i) PCl5 is more covalent than PCl3.
(ii) Iron on reaction with HCl forms FeCl2 and not FeCl3.
(iii) The two O-O bond lengths in the ozone molecule are equal.
Answer. (i) In PCl5, phosphorus has +5 oxidation state and has less tendency to loose electrons than in +3 of PCl3. Therefore, PCl5 has more tendency to share e–1s than PCl3.
(ii) Because HCl on reaction with iron liberates H2 gas which prevents the formation of ferric chloride.
Question. (a) Arrange the hydrides of group 16 in increasing order of their acidic character. Justify your answer.
(b) Draw structure of XeOF4.
Answer.
(a) H2O < H2S < H2Se < H2Te
As we move down the group the bond dissociation enthalpy decreases due to increase in bond length and size of the central atom.
Question. Account for the following :
(i) Sulphur in vapour form exhibits paramagnetic behaviour.
(ii) SnCl4 is more covalent than SnCl2.
(iii) H3PO2 is a stronger reducing agent than H3PO3.
Ans (i) In vapour state sulphur partly exists as S2 molecule which has two unpaired electrons in the antibonding II orbitals and hence exhibits paramagnetism.
(ii) Sn+4 in SnCl4 has more polarising power than SnCl2
(iii) H3PO2 contains two P-H bonds while H3PO3 contains only one P-H bond therefore H3PO2 is stronger reducing agent.
Long Answer Type Questions
Question. (a) Draw the structures of the following molecules :
(i) (HPO3)3 (ii) BrF3
(b) Complete the following chemical equations :
(i) HgCl2 + PH3 → (ii) SO3 + H2SO4 →
(iii) XeF4 + H2O →
Answer.

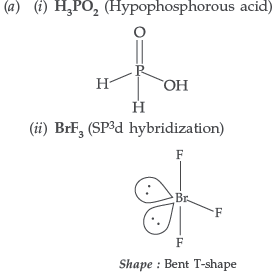
Question. (a) Draw the structures of the following :
(i) H3PO2 (ii) BrF3
(b) How would you account for the following observations :
(i) Phosphorus has a greater tendency for catenation than nitrogen.
(ii) Bond dissociation energy of fluorine is less than that of chlorine.
(iii) No chemical compound of helium is known.
Answer.
(b) (i) The bond strength of P–P is more than N–N, therefore phosphorous shows more tendency for catenation than nitrogen.
(iii) Because the ionization energy of Helium is very high and the empty d-orbitals are also absent in it.
Question. (a) Draw the structures of the following :
(i) XeF4 (ii) H2S2O7
(b) Explain the following observations :
(i) Phosphorus has a greater tendency for catenation than nitrogen.
(ii) The negative value of electron gain enthalpy is less for fluorine than that for chlorine.
(iii) Hydrogen fluoride has a much higher boiling point than hydrogen chloride.
Answer.

(b) (i) The bond strength of P–P is more than N–N, therefore phosphorous shows more tendency for catenation than nitrogen.
(ii) Because of small size of flourine atom and strong electron–electron repulsions in its compact 2p orbitals.
(iii) Hydrogen fluoride (HF) has higher boiling point than HCl due to extensive intermolecular hydrogen bonding while HCl doesn’t show this H-bonding.
Question. (a) Explain the following :
(i) NF3 is an exothermic compound whereas NCl3 is not.
(ii) F2 is most reactive of all the four common halogens.
(b) Complete the following chemical equations :
(i) C + H2SO4 (conc) →
(ii) P4 + NaOH + H2O →
(iii)Cl2 + F2 →
(excess)
Answer.
(ii) Because of the low bond dissociation energy F2 readily dissociates into atoms and reacts with other substances readily.
(b) (i) C + 2H2SO4 (conc.) → CO2 + 2SO2 + 2H2O
(ii) P4 + 3NaOH + 3H2O → PH3 + 3NaH2PO2
(iii) Cl2 + 3F2 → 2ClF3
(excess)
Question. (a) Draw the structures of the following :
(i) PCl5 (s) (ii) SO32–
(b) Explain the following observations :
(i) Ammonia has a higher boiling point than phosphine.
(ii) Helium does not form any chemical compound.
(iii) Bi (V) is a stronger oxidising agent than Sb (V).
Answer.

Angle : The angle O – S – O is greater than 90°
(b) (i) Due to intermolecular H-bonding in NH3 it has higher boiling point than PH3 which does not have any H-bonding.
(ii) Because the ionization energy of Helium is very high and very high positive electrons gain enthalpy.
Question. (a) Complete the following chemical equations :
(i) NaOH (aq) + Cl2 (g) →
(Hot and conc.)
(ii) XeF6 (s) + H2O(l) →
(b) How would you account for the following?
(i) The value of electron gain enthalpy with negative sign for sulphur is higher than that for oxygen.
(ii) NF3 is an exothermic compound but NCl3 is endothermic compound.
(iii) ClF3 molecule has a T-shaped structure and not a trigonal planar one.
Answer. (a) (i) 6NaOH + 3Cl2→ 5NaCl + 1NaClO3 + 3H2O
(ii) XeF6 (s) + 3H2O (l) ⎯ → XeO3 (s) + 6HF (aq)
(b) (i) Because of enthalpy of dissociation of S – S bond is higher than O – O bond and the hydration energy of S2– is less than that of O2– ion.
(ii) Due to smaller size of F as compared to Cl, the N – F bond is much stronger than N– Cl bond while bond dissociation energy of F2 is much lower than that of Cl2. Therefore, energy released during the formation of NF3 molecule is more than the energy needed to break N2 and F2 molecules into individual atoms. In other words, formation of NF3 is an exothermic reaction.
The energy released during the formation of NCl3 molecule is less than the energy needed to break N2 and Cl2 molecule into individual atoms. Thus formation of NCl3 is an endothermic reaction.
(iii) The electronic configuration of Cl is 1s2 2s2 2p6 3s2 3P2x 3P2y 3P1z x . It has only one half filled orbital. But to form three Cl – F bonds, we need three half filled orbitals.To achieve this, one of the 3P2y electrons gets excited to 3d orbital. The resulting five orbitals of the third shell of Cl in the first excited state undergoes SP3d hybridization to give T-shaped structure to ClF3 molecule. Since Cl does not undergo SP2 hybridization, therefore ClF3 does not have trigonal planar structure.

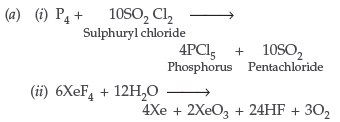
Question. (a) Complete the following chemical reaction equations :
(i) P4 + SO2Cl2 ⎯⎯→
(ii) XeF4 + H2O ⎯⎯→
(b) Explain the following observations giving appropriate reasons :
(i) The stability of +5 oxidation state decreases down the group in group 15 of the periodic table.
(ii) Solid phosphorus pentachloride behaves as an ionic compound.
(iii) Halogens are strong oxidizing agents.
Answer.
(b) (i) Group 15 first elements are pentavalent, therefore they can show positive oxidation state +3 (due to P-electron) and +5 (due to P and S electrons). In a group the +5 oxidation state stability decreases but +3 oxidation state increases due to inter pair effect which results the 5- orbitals electrons to participate in bonding hence shows +3 oxidation state as their stable oxidation state.
(ii) PCl5 conducts electricity in the molten state. This means that in solid state it exists as [PCl4]+ [PCl6]– in which the cation is tetrahedral and the anion is octahedral.
2PCl5 → [PCl4]+ [PCl6]–
On melting, these ions become free to move and hence PCl5 conducts electricity in the molten state.
(iii) As halogens are strong elctron acceptors and change to negative ions and thus undergo reduction, so they are strong oxidising agent.
Question. (a) Draw the structures of the following :
(i) H2S2O8 (ii) HClO4
(b) How would you account for the following :
(i) NH3 is a stronger base than PH3.
(ii) Sulphur has a greater tendency for catenation than oxygen.
(iii) F2 is a stronger oxidising agent than Cl2.
Answer. (a) (i) H2S2O8 (Peroxodisulphuric acid) or Marshall’s acid :
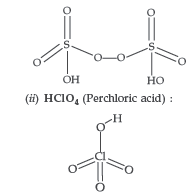
(iii) Due to low bond dissociation enthalpy and high electronegativity of Fluorine, it has strong tendency to accept electrons and thus get reduced.
F + e– → F–
Therefore F2 acts as strong oxidising agent, while Cl2 is weak oxidising agent due to low electronegativity.
Question. (a) Complete the following chemical equations :
(i) Cu + HNO3 (dilute) →
(ii) XeF4 + O2F2 →
(b) Explain the following observations :
(i) Phosphorus has greater tendency for catenation than nitrogen.
(ii) Oxygen is a gas but sulphur a solid.
(iii) The halogens are coloured. Why?
Answer. (a) (i) 3Cu + 8HNO3 (dil) → 3Cu(NO3)2 + 2NO + 4H2O
(ii) XeF4 + O2F2 → XeF6 + O2
(b) (i) The self linking property or catenation property of nitrogen is less than that of phosphorus because N – N bond is weaker than P – P bond.
(ii) Oxygen forms a stable diatomic molecule. In O2 molecule two atoms of oxygen have joined together through double bond O = O. The multiple bonding is possible due to small size of oxygen atoms. So oxygen is a gas. S has eight atoms arranged in the form of a puckered ring per molecule in which two sulphur atoms are joined by covalent bonds. So sulphur is a solid at room temperature.
(iii) All halogens are coloured. This is due to absorption of radiation in visible region which results in the excitation of outer electrons to higher energy level. So they display different colours.
Question. (a) Account for the following :
(i) The acidic strength decreases in the order HCl > H2S > PH3
(ii) Tendency to form pentahalides decreases down the group in group 15 of the periodic table.
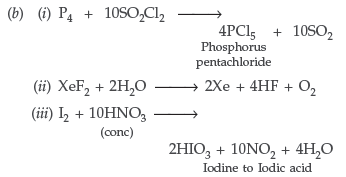
(b) Complete the following chemical equations :
(i) P4 + SO2Cl2 →
(ii) XeF2 + H2O →
(iii) I2 + HNO3 →
(conc)
Answer. (a) (i) Because of decrease in electronegativity from chlorine to phosphorous, the bond dissociation enthalpy from HCl to H–P increases and their tendency to release
H+ decreases and thus acidic strength decreases.
(ii) Down the group, the tendency of next ‘s’ orbital’s electron to jump to previous ‘d’ orbital decreases very much due to inert pair effect.
Question. (a) Draw the structures of the following :
(i) H2S2O7 (ii) HClO3
(b) Explain the following observations :
(i) In the structure of HNO3, the N –O bond (121 pm) is shorter than the N – OH bond (140 pm).
(ii) All the P – Cl bonds in PCl5 are not equivalent.
(iii) ICl is more reactive than I2.
Answer. (a) (i) H2S2O7 (Pyrosulphuric acid) or oleum :

(b) (i) The N – O bond has partial double bond character while the N – OH bond is a single bond in both resonance of HNO3.
(ii) All the P – Cl bonds in PCl5 are not equivalent due to the fact that the axial bond pairs suffer more repulsion as compared to equatorial bond pairs.
(iii) Because ICl bond is weaker than I – I bond as a result of which ICl breaks easily to form halogen atoms which readily bring about the reaction, hence more reactive.
Question. (a) What happens when
(i) chlorine gas is passed through a hot concentrated solution of NaOH?
(ii) sulphur dioxide gas is passed through an aqueous solution of a Fe (III) salt?
(b) Answer the following :
(i) What is the basicity of H3PO3 and why?
(ii) Why does fluorine not play the role of a central atom in interhalogen compounds?
(iii) Why do noble gases have very low boiling points?
Answer.
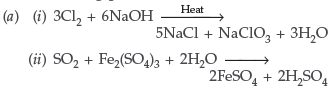
(b) (i) Basicity of H3PO3 = 2
Because basicity is the number of replaceable H+ ions in an acid and H3PO3 is a Dibasic acid.
(iii) Because the atoms of these elements are held together by weak van der Waal’s forces of attraction.
Question. (a) Complete the following chemical reaction equations :
(i) P4 + SO2Cl2 →
(ii) XeF6 + H2O →
(b) Predict the shape and the asked angle (90° or more or less) in each of the following cases :
(i) SO32− and the angle O – S – O
(ii) ClF3 and the angle F – Cl – F
(iii) XeF2 and the angle F – Xe – F
Answer.
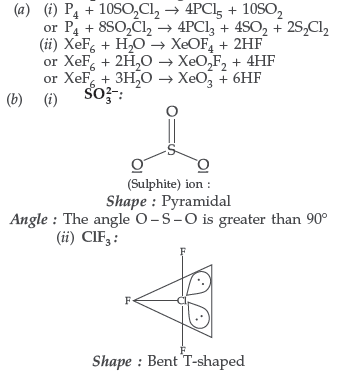
Question.150. Account for the following :
(a) Thermal stability of water is much higher than that of H2S.
(b) White phosphorus is more reactive than red phosphorus.
(c) Ammonia acts as a ligand.
(d) Bismuth is a strong oxidizing agent in pentavalent state.
(e) Concentrated sulphuric acid is a strong dehydrating agent.
Answer. (a) The thermal stablility of hydrides decrease from H2O to H2S because as the size of the atom increases, the bond becomes weaker and thus breaks on heating.
(b) Red phosphorus is regarded as a polymer consisting of chains of P4 tetrahedral linked together. This makes phosphorus denser and less reactive

But in white phosphorus (P4 ), the four phosphorus atoms lie at the corners of a regular tetrahedron. Each phosphorus atom is linked to each of the other three atoms by covalent bond. The bond angle is equal to 60o which suggests that the molecule is under strain and hence active in nature.

(c) Due to the presence of a lone pair of electrons on nitrogen atom, it has a tendency to donate an electron pair, hence acts as a ligand.
(d) In Bismuth, the inert pair effect is very prominent. Thus +5 oxidation state is less stable in comparison to +3 oxidation state i.e it readily accepts two electrons in pentavalent state and gets reduced to trivalent state. Therefore, it acts as a strong oxidising agent.
(e) Conc. H2SO4 has great affinity for water molecule i.e it acts as a dehydrating agent.
Question. (a) Draw the molecular structures of the following compounds :
(i) N2O5 (ii) XeOF4
(b) Explain the following observations :
(i) Sulphur has a greater tendency for catenation than oxygen.
(ii) ICl is more reactive than I2.
(iii) Despite lower value of its electron gain enthalpy with negative sign, fluorine (F2) is a stronger oxidising agent than Cl2.
Answer.
(b) (i) It is because S – S bond is stronger than O – O bonds as there is more interelectronic repulsion in O – O due to small size than in S – S.
(ii) ICl is more reactive than I2 because I-Cl bond is polar and weaker while I – I bond is stronger and non-polar.
(iii) It is due to (a) low enthalpy of dissociation of F – F bond, (b) high hydration enthalpy of F.
