Chapter 1 Chemical Reactions and Equations Class 10 Science Notes
Students should read Chapter 1 Chemical Reactions and Equations Class 10 Science Notes provided below. These notes have been prepared based on the latest syllabus and books issued by NCERT, CBSE and KVS. These important revision notes will be really useful for students to understand the important topics given in the chapter Chemical Reactions and Equations in Class 10 Science. We have provided class 10 science notes for all chapters.
Revision Notes Chapter 1 Chemical Reactions and Equations Class 10 Science
Chapter 1 Chemical Reactions and Equations is an important chapter in Class 10 Science. The following notes will help you to understand and easily learn all important points to help you score more marks.
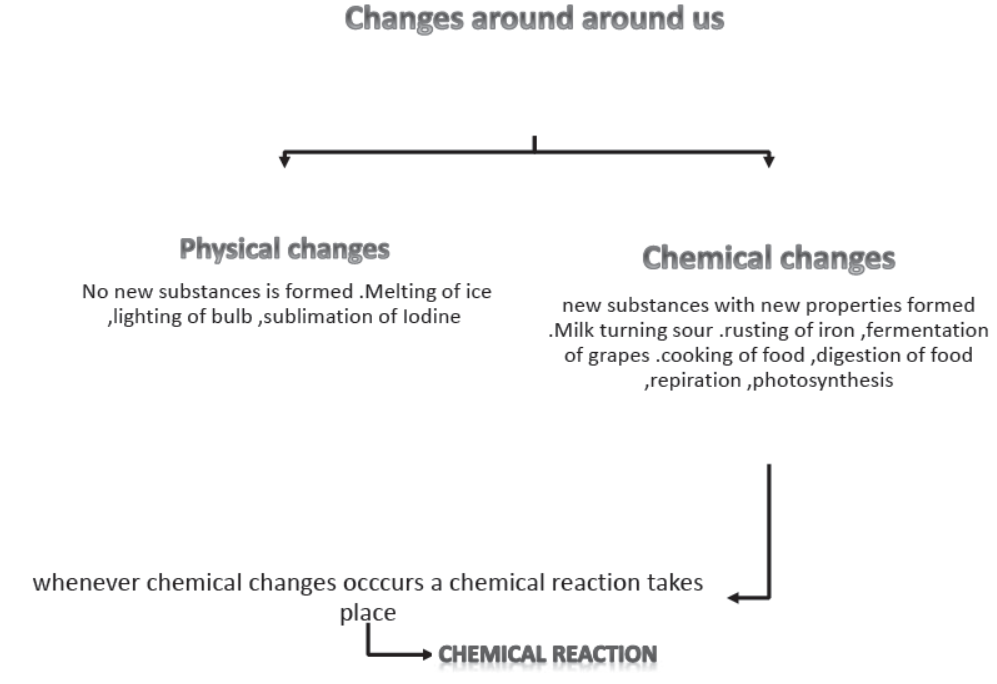
1. Valency. The number of outermost electrons shared by an atom is called its valency. It is also called the combining capacity of an atom, e.g. Cl atom can share one valence electron, its alency is l, Oxygen can share two valence electrons, and its valency is 2. Nitrogen can share 3 valence electrons, its valency is 3, Carbon can share 4 valence electrons, and therefore its valency is 4 and so on. It means if Carbon combines with Chlorine, Carbon will share four valence electron with four Chlorine atoms, therefore the molecular formula of the covalent compound will be
2.A chemical reaction is represented by a Chemical Equation:
Chemical Equations. “A chemical equation is a symbolic notation that uses formulae of compounds and symbols of elements to represent a chemical reaction”, e.g. Copper oxide reacts with Carbon to form Copper and Carbon monoxide. The reaction may be represented as
CuO + C → Cu + CO
(Copper oxide) (Carbon) (Copper) (Carbon monoxide)
2. Writing of a Chemical Equation.
I. The symbols of elements and the formulae of reacting substances (reactants) are written on the left hand side and plus (+) sign is written between them.
II. The symbols and formulae of the substances formed (products) are written on the right hand side with a plus sign (+) between them.
III. An arrow (→) sign is put between the reactants and products, e.g.,
Magnesium + Sulphuric acid→ Magnesium sulphate + Hydrogen gas
Mg + H2SO4 → MgSO4 + H2
IV. The physical states of the reactants and products are also mentioned in a chemical equation. The notations g, l, s, aq. Are written in brackets along with symbols/formulae of reactants and products. These symbols stand for gaseous liquid, solid and aqueous solution respectively, e.g.,
Mg(s) + H2SO4(aq) → MgSO4(aq) + H2(g)
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2
Zinc Sulphuric acid
The symbol (↑) may also be used to represent a gaseous product. The symbol (↓) is used to represent the formation of a precipitate (water insoluble) or a sparingly soluble substance formed during the reaction which generally settles down, e.g. ,
NaCl(aq) + AgNO3(aq) → AgCl(↓) + NaNO3(aq)
Sodium chloride Silver nitrate Silver chloride Sodium nitrate
V. Sometimes the temperature, pressure and catalyst of the reaction are indicated above or below the arrow in the equation, e.g. ,
CO(g) + 2H2(g) → ZnO/Cr2O3 340 atm,heat CH3OH(g)
Carbon monoxide Hydrogen Methanol
VI. A chemical equation represents an actual chemical reaction in which the reactants and products are known e.g. ,
2KMnO4(s) heat→ K2MnO4(s) + MnO2(s) + O2(g)
Potassium Potassium Manganese Oxygen
Permanganate manganite dioxide
2KClO3(s) heat → MnO2 2KCl(s) + 3O2(g)
Potassium chlorate Potassium chloride Oxygen
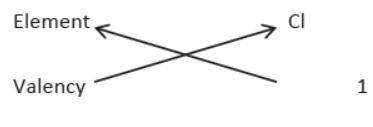
3. Balanced Equations. The equations in which atoms of various elements on the reactants and the products side are equal. Given equation is not balanced because the number of hydrogen atoms is not equal on both sides. It is called a skeleton or unbalanced chemical equation.
Na + H2O → NaOH + H2
4. Balancing of Chemical Equation. Observe given chemical equation:
Zn + H2SO4 → ZnSO4 + H2
In this equation the number of atoms of Zn, H, S and O are equal on both sides, i.e. the equation is balanced.
5. Reason of Balancing Equation. The number of atoms of elements on both sides of a chemical equation should be equal in accordance with the law of conservation of mass.
6. Types of Chemical Reactions.

The chemical reactions are classified into various categories depending upon the types of changes taking place. The different types of reactions are as follows:
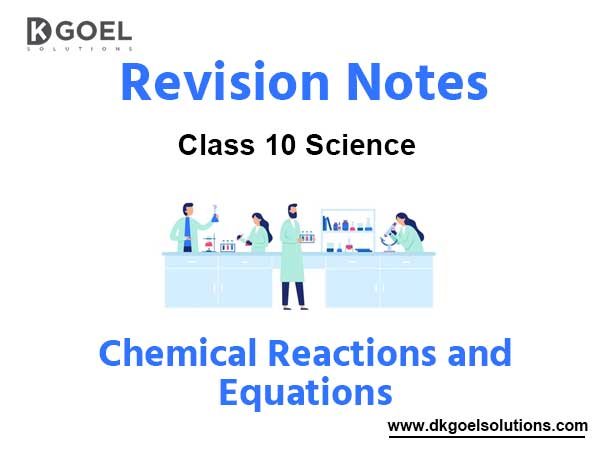
1. Combination Reactions. Those reactions in which two or more substances combine to form new substance(s) are called combination reactions.
2. Decomposition reaction. A reaction in which a single compound breads down to produce two or more simpler substances, i.e., a compound decomposes into simpler substances.
It is opposite to combination reactions.
There are three ways in which decomposition reactions can be carried out, i.e., energy
required in decomposition reaction can be supplied in the following ways:
a. Heat
b. Electricity
c. Light
(a) Thermal Decomposition (Heat). When decomposition reaction is carried out by heating it is called thermal decomposition reaction, e.g.,
CaCO3(s) heat→ CaO(s) + CO2(g)
Calcium carbonate Quick lime
ZnCO3(s) heat → ZnO(s) + CO2(g)
Zinc carbonate Zinc oxide
The Process of heating ZnCO3 (Calamine), an ore of zinc in absence of air to form Zinc oxide (ZnO) and CO2(g) is also called calcination.
(b) Electrolysis. When decomposition reaction is carried out with the help of electric current, the process is called electrolysis (‘electro’ means electric ‘lysis’ means break down), e.g. when electric current is passed through acidified water (water mixed with a few drops of acid so as to make it a good conductor), it decomposes into Hydrogen and Oxygen gases.
2H2O(l) Electric current → 2H2(g) + O2(g)

(c) Photochemical Decomposition Reaction. When decomposition reaction is carried out in the presence of sunlight, the process is called photochemical decomposition. e.g
2AgBr(s) Sunlight → 2Ag(s) + Br2(g).
Silver bromide Siler Bromine
(iii) Displacement Reactions. Those reactions in which more reactive element can displace less reactive element to form a compound are called displacement reaction for example:
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
Zinc (grey) Copper sulphate (Blue) (colourless) Copper (reddish brown)
Mg(s) + ZnSO4(aq) → MgSO4(aq) + Zn(s) Zinc sulphate (Colourless)
(Magnesium sulphate) + Zinc
(Colourless) (grey)
Zn(s) + FeSO4(aq) → ZnSO4(aq) + Fe(s)
Pale green Colourless Black
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
Colourless Blue Shiny silver
(iv) Double displacement reactions. Those reactions in which two different atoms or group of atoms are displaced by other atoms or group of atoms, i.e., two compounds exchange their ions and one of the products formed is insoluble.
(v) Neutralization Reactions. Those reactions in which acid or acidic oxide reacts with base or basic oxide to form salt and water are called neutralization reactions, e.g.
NaOH(aq) + HNO3(aq) → NaNO3(aq) + H2O(l)
2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(l)
KOH(aq) + HCL(aq) → KCl(aq) + H2O(l)
KOH(aq) + HNO3(aq) → KNO3(aq) + H2O(l)
2KOH(aq) + H2SO4(aq) → K2SO4(aq) + 2H2O(l)
CH3COOH(aq) + NaOH(aq) → Ch3COONa(aq) + H2O(l)
(vi) Oxidation and Reduction.
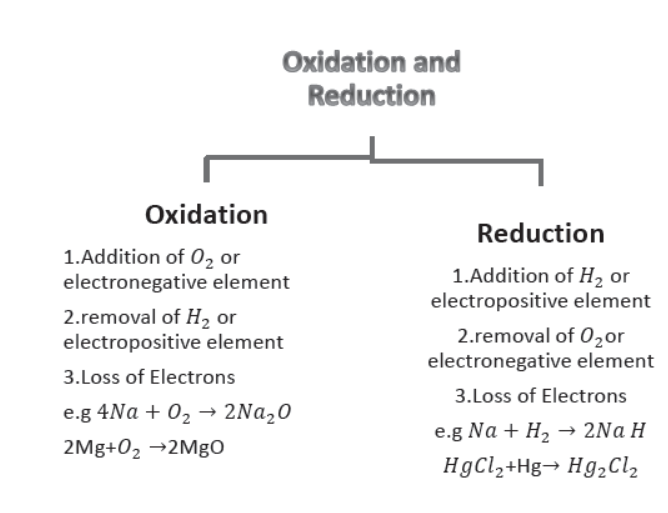
(a) Oxidation. It is a process in which Oxygen or an electronegative element is added.
It can also be defined as a process in which Hydrogen or an electropositive element is removed.
In terms of electronic concept. Oxidation is a process in which loss of electrons takes place e.g.
4Na(s) + O2(g) → 2Na2O(s) (Addition of Oxygen)
Sodium oxide
2Mg(s) + O2(s) → 2MgO(s) (Addition of Oxygen)
Magnesium oxide
(b) Reduction. It is process in which addition of Hydrogen or an electropositive element takes place.
It is also defined as a process in which Oxygen or an electronegative element is removed.
In electronic concept reduction process involves gain of electrons.
2Na(s) + H2(g) → 2NaH(s) (Addition of Hydrogen)
Sodium hydride
CuO(s) + H2(g) → Cu(s) + H2O(l) (Removal of Oxy. & addition of Hydrogen)
Copper oxide
Fe3+ + e– → (aq) + Fe2+ (aq) (Gain of electron)
Hg(l) + HgCl2(aq) → Hg2Cl2(aq) (Addition of an electropositive element)
Cu(s) + CuCl2(aq) → Cu2Cl2(aq) (Addition of an electropositive element)
Cupric chloride cuprous chloride
AuCl3(aq) → AuCl(aq) +Cl2(g) (Removal of an electronegative element)
Auric chloride
Gold (III) Chloride Aurous chloride Chlorine
(c) Redox Reactions. Those reactions in which oxidation and reduction take place simultaneously are called redox reactions, e.g.


Fe is losing electrons therefore it acts as a Reducing agent.
S is gaining electrons therefore, it acts as an Oxidising agent.
Fe is getting oxidized to Fe2+ [Iron(II) ion] whereas S gets reduced to S2- (Sulphide ion)
8. Corrosion (Rusting).
*Surface of metal is attacked by air water and other
substance it is redox reaction
Prevention:
1.Painting
2.oiling
3.gressing
3.Galvanisation
4.elctroplating
condition of rusting :
1.Prescense of water
2.Oxygen and acid
The metal which is reactive, its surface is attacked by air, water and other substances around it is said to corrode and the process is called corrosion. It is redox reaction because metal gets oxidised to metal oxide and oxygen gets reduced to oxide ion.
Rust is mainly hydrated iron(III) oxide Fe2O3-xH2O. Rusting wakens the structure of body of vehicles, bridges, iron railing, etc. Corrosion of iron is serious problem because it leads to wastage of tones of iron every year and lot of money is spent to repair or replace
9.Rancidity.
*the spoilage of food by oxidation .It smells ,tatse ,
changes ,called rancidity
Prevention:
1.Antioxidants are added.
2.air tight container prevent oxidation
3.chips and snacks packs filled with nitrogen
When fats and oils are oxidised, they become rancid and their smell and taste changes.
To prevent spoilage of food special type of substances called antioxidants are added to fatty foods to prevent oxidation e.g. chips are packed in nitrogen gas which prevent spoilage of chips by oxidation.
Oxidation process can be slowed down by reducing the temperature. The food is kept in refrigerator to slow down the process of oxidation.
The food can be kept in air tight container so as to prevent them from oxidation