Exam Question for Class 10 Science Chapter 1 Chemical Reactions and Equations
Please refer to below Exam Question for Class 10 Science Chapter 1 Chemical Reactions and Equations. These questions and answers have been prepared by expert Class 10 Science teachers based on the latest NCERT Book for Class 10 Science and examination guidelines issued by CBSE, NCERT, and KVS. We have provided Class 10 Science exam questions for all chapters in your textbooks. You will be able to easily learn problems and solutions which are expected to come in the upcoming class tests and exams for standard 10th.
Chapter 1 Chemical Reactions and Equations Class 10 Science Exam Question
All questions and answers provided below for Exam Question Class 10 Science Chapter 1 Chemical Reactions and Equations are very important and should be revised daily.
Exam Question Class 10 Science Chapter 1 Chemical Reactions and Equations
Very Short Answer Type Questions
Question: Give an example of decomposition reaction taking place in our body.
Answer: An example of decomposition reaction taking place in our body is digestion of food. When we eat foods like wheat, rice or potatoes, then the starch present in them decomposes to give simple sugars like glucose in the body and the proteins decompose to form amino acids.
Question: Why are anti-oxidants added to fat and oil containing food items?
Answer: Anti-oxidants are added to fat and oil containing food items to prevent their oxidation which is known as rancidity which makes food smell bad
and changes its taste.
Question: An aqueous solution of metal nitrate P reacts with sodium bromide solution to form yellow ppt of compound Q which is used in photography. Q on exposure to sunlight undergoes decomposition reaction to form metal present in P along with reddish brown gas. Identify P & Q.
Answer: As the compound Q is formed by the reaction between P (metal nitrate solution) and sodium bromide solution, Q is silver bromide which is used in photography.
Further Q undergoes decomposition in the presence of sunlight to form silver and bromine gas. As silver is formed above, the metal nitrate is silver nitrate.
P – silver nitrate (AgNO3)
Q – silver bromide (AgBr).
Question: A substance X used for coating iron articles is added to a blue solution of a reddish brown metal Y, the color of the solution gets discharged Identify X and Y & also the type of reaction.
Answer: We are given that the metal Y is reddish brown in colour. This metal is copper and the blue solution of Y is copper sulphate. Also, zinc is used in protecting iron articles and this process is called galvanisation. As Zinc is more reactive than copper it displaces copper from its salt solution, copper sulphate.
Zn + CuSO4 → ZnSO4 + Cu
So, X is zinc, while Y is copper.
Question: In this reaction mention the substance getting oxidized and the substance getting reduced.
Fe2O3 + 2Al → Al2O3 + 2Fe
Answer: In the reaction given, Iron oxide (Fe2O3) loses oxygen to form Fe. Therefore, Iron oxide is getting reduced.
Aluminium (Al) gains oxygen to form Aluminium oxide (Al2O3). Therefore, Aluminium is getting oxidized.
Question: A light sensitive compound “X” of silver is used in black and white photography. On exposure to sunlight its colour changes to gray. Identify “X” and write a chemical equation to express the above change.
Answer: X is silver chloride as it is used in black and white photography and on exposure to sunlight undergoes decomposition reaction to form silver (grey) and chlorine gas.
Chemical equation of the reaction taking place is:
2AgCl(s) Sunlight → hr 2Ag(s) + Cl2(g)
SHORT ANSWER
Question: When hydrogen sulphide gas is passed through a blue solution of copper sulphate, the colour of the solution fades and a black precipitate is obtained.
(A) Name the type of reaction mentioned above.
(B) Why does the colour of the solution fade away?
(C) Write the chemical name of the black precipitate formed.
(D) Give the balanced chemical equation for the reaction involved.
Answer: When hydrogen sulphide gas is passed a blue solution of copper sulphate, the blue colour of the solution fades and a black precipitate of copper sulphide is formed alongwith sulphuric acid.
(A) The type of the reaction mentioned above is double displacement reaction.
Explanation: In this double displacement reaction, two compounds, hydrogen sulphide gas combines with copper sulphate solution react to form two new compounds, copper sulphide and sulphuric acid. An exchange of ions takes place in this reaction For example (Cu2+) copper ions of copper sulphate solution react with sulphide ions (S2–) of hydrogen sulphide to form a new compound copper sulphide (Cu2+ S2– or CuS). Similarly the hydrogen ions (H+) of hydrogen sulphide react with sulphate ions (SO4 2–) of copper sulphate to form new compound, sulphuric acid (2H+ SO4 2–) compound. Copper Sulphide (CuS) is formed as an insoluble black precipitate. So is also called precipitation reaction.
(B) The blue colour of copper sulphate fades due to its reaction with hydrogen sulphide gas and results into the formation of colourless solution of sulphuric acid.
(C) The chemical name of black precipitate is copper sulphide.
(D) H2S(g) + CuSO4(aq) → CuS(s)↓ + H2SO4(aq)
Hydrogen Copper Copper Sulphuric
Sulphide sulphate Sulphide acid
gas solution (Black)
(Blue)
Question: Why do fire flies glow at night?
Answer: Fire flies have a luminous bag in their lower abdomen region that contains a protein called luciferin. This luminous bag also secretes a certain enzyme called luciferare. On reaction with luciferin, this enzyme emits light in the presence of magnesium and oxygen. Therefore, fireflies glow at night.
Question: Which among the following are physical or chemical changes?
(A) Evaporation of petrol
(B) Burning of Liquefied Petroleum Gas (LPG)
(C) Heating of an iron rod to red hot
(D) Curdling of milk
(E) Sublimation of solid ammonium chloride
Answer: (A) This is a physical change. Petrol only changes its state from the liquid form to the gaseous form because of the presence of energy in the form of heat. A change of state is a reversible reaction and hence, this is a physical change.
(B) This is a chemical change. Burning of liquefied petroleum gas releases energy and forms CO2 and H2O as its by products which cannot be converted back to the original reactant. The chemical composition of LPG has changed. Hence, it is a chemical change.
(C) This is a physical change. The temperature affects the metal temporarily. On reversing the temperature change, the metal goes back to its previous change. Hence, it is a physical state.
(D) This is a chemical change. The process of curdling of milk takes place due to bacteria.
It is an irreversible reaction in which the composition of milk is changed and hence, is a chemical change.
(E) This is a physical change. Energy in the form of heat has prompted solid ammonium chloride to evaporate. On reversing the temperature, it goes back to its previous solid state. Hence, this is a physical change.
Question: During the reaction of some metals with dilute hydrochloric acid, the following observations were made:
(A) Silver metal does not show any change.
(B) The temperature of the reaction mixturerises when aluminium (Al) is added.
(C) The reaction of sodium metal is found to be highly explosive.
(D) Some bubbles of a gas are seen when lead
(Pb) reacts with the acid. Explain these observations giving suitable reasons.
Answer: (A) Silver is less reactive than hydrogen and hence, cannot displace hydrogen in a chemical reaction. (silver lies below hydrogen in the activity series).
(B) The temperature of the reaction mixture rises when aluminium (Al) is added to HCl because following exothermic reaction takes place:
2Al + 6HCl → 2AlCl3 + 3H2 + Heat
(C) Sodium is a very reactive metal. The reaction of sodium metal with dilute hydrochloric acid is so vigorously exothermic that evolving hydrogen gas catches fire and explodes produced catches fire immediately.
(D) The bubbles are observed due to the formation of Hydrogen gas. The reaction
gradually becomes slow due to the formation of a coating of Lead chloride on Lead, which prevents further reaction.
Pb + 2HCl → PbCl2 + H2(g)
Question: Write a balanced chemical equation for each of the following reactions and also classify them:
(A) Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic acid solution.
(B) A piece of sodium metal is added to absolute ethanol to form sodium ethoxide
and hydrogen gas.
(C) Iron (III) oxide on heating with carbon monoxide gas reacts to form solid iron and liberates carbon dioxide gas.
(D) Hydrogen sulphide gas reacts with oxygen gas to form solid sulphur and liquid water.
Answer: (A) Pb(CH3COO)2(aq) + 2HCl(dil) → PbCl2(s)↓ + 2CH3COOH(aq)This is a double displacement (precipitation reaction).
(B) 2C2H5OH(l) + 2Na(s) → 2C2H5O−Na+ + H2↑ This is a displacement reaction.
(C) Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)↑ This is a redox reaction.
(D) 2H2S(g) + O2(g) → 2S(s) + 2H2O(l) This is a redox reaction.
Question: A magnesium ribbon is burnt in oxygen to give a white compound X accompanied by emission of light. If the burning ribbon isnow placed in an atmosphere of nitrogen, it continues to burn and forms a compound Y.
(A) Write the chemical formulae of X and Y.
(B) Write a balanced chemical equation for
when X is dissolved in water.
Answer: (A) When magnesium ribbon is burnt in oxygen, it forms magnesium oxide with emission of light and heat energy.
2Mg(s) + O2(g) Heat → 2MgO(s) + Energy
Magnesium Oxygen Magnesium oxide ‘X’
The chemical formula of X is MgO.
If the burning ribbon is placed in a nitrogen gas chamber, magnesium reacts with nitrogen and forms magnesium nitride (Mg3N2).
3Mg(s) + N2(g) Heat → Mg3N2(s)
Magnesium Nitrogen Magnesium
nitride ‘Y’
The chemical formula of compound Y is Mg3N2.
(B) If magnesium oxide is dissolved in water, it forms magnesium hydroxide.
MgO(s) + H2O(l) → Mg(OH)2(aq)
Magnesium Water Magnesium
oxide ‘X’ hydroxide
Question: What is the general name of chemicals which are added to fat and oil containing food to prevent the development of rancidity?
Answer: The chemicals that are added to fat and oil- containing food to prevent the development of rancidity are called antioxidants. These substances prevent the oxidation of fats and oils and so prevent bad odour from being formed in the food.
Question: On adding a drop of barium chloride solution to an aqueous solution of sodium sulphite, white precipitate is obtained.
(A) Write a balanced chemical equation of the reaction involved.
(B) What other name can be given to this precipitation reaction?
(C) On adding dilute hydrochloric acid to
the reaction mixture, white precipitate
disappears. Why?
Answer: (A) Na2SO3 (aq) + BaCl2 (aq) → BaSO3 (s) + 2NaCl (aq)
(B) Double displacement reaction
(C) On adding dilute hydrochloric acid to the reaction mixture, white precipitate
disappears. This is because addition of HCl dissolves the white precipitate of BaSO3 and form soluble BaCl2.
BaSO3(s) + 2HCl(aq) → BaCl2(aq) + H2O(l) + SO2(g)
Question: (A) Mention any four information given by a chemical equation.
(B) State the law of conservation of mass as
applicable in a chemical reaction.
Answer: (A) A balanced chemical equation tells:
(1) number of atoms and molecules of reactants and products involved.
(2) chemical formula of reactants and products involved.
(3) catalyst involved in the reaction if any.
(4) physical state of reactants and products involved.
Question: In the electrolysis of water:
(A) Name the gases liberated at anode and cathode.
(B) Why is it that the volume of gas collected on one electrode is two times that on the other electrode?
(C) What would happen if dil. H2SO4 is not added to water?
Answer: (A) Gas liberated at anode – Oxygen Gas liberated at Cathode–Hydrogen
(B) On electrolysis water decomposes into hydrogen and oxygen:
2H2O(l) Electric → Current 2H2(g) + O2(g)
Water decomposes to hydrogen and oxygen in the ratio of 2 : 1 by volume. The double volume collected is hydrogen.
(C) If dilutes sulphuric acid is not added to water, electrolysis will not take place as water is not a good conductor, if distilled water is used. Otherwise otherwise also conductivity will be very low if tap water is used. Sulphuric acid is added to water to make it a good conductor.
Question: (A) Write two observations when lead nitrate is heated in a test tube.
(B) Name the type of reaction.
(C) Write a balanced chemical equation to represent the above reaction.
Answer: (A) It turns yellow due to formation of lead oxide and Reddish brown fumes evolve.
(B) Thermal decomposition reaction.
(C) 2Pb(NO3)2 Heat → 2PbO + 4NO2 + O2
Question: Translate the following statements into chemical equations and balance them (if needed):
(A) Lead nitrate undergoes thermal decomposition to form lead monoxide, nitrogen dioxide and oxygen gas.
(B) Quicklime combines with carbon dioxide to form calcium carbonate.
(C) Aluminium metal granule is added in sulphuric acid to form aluminium sulphate and hydrogen gas.
Answer:

Question: No chemical reaction takes place when granules of a solid A are mixed with the
powder of another solid B. However when the mixture is heated, a reaction takes place between its components. One of the products, C, is a metal and settles down in its molten state, while the other product, D, floats over it. It was observed that the reaction is highly exothermic.
(1) Based on the given information, make an assumption about A and B and write
a chemical equation for the chemical reaction indicating the conditions of the reaction, physical state of reactions and products and thermal status of reaction.
(2) Mention any two types of reaction under which the above chemical reaction can be classified.
Answer: Here, A is solid manganese granules while B is aluminium powder. When they are mixed together, no reaction takes place. However, on heating, thermite reaction takes place. Here, the aluminium takes the oxygen from the manganese dioxide to form molten metallic manganese. It also results in the formation of aluminium oxide which floats over manganese metal. The chemical reaction is as follows:
3MnO2(s) + 4Al(s) Δ→ 3Mn(l) + 2Al2O3(s)
Manganese Aluminium Metal Aluminium
dioxide manganese oxide
Since this chemical reaction releases a lot of heat (exothermic reaction), it results in oxidation of aluminium and reduction of manganese.
Hence, it is also a redox reaction.
Question: (A) Hydrous ferrous sulphate crystals are heated in a boiling tube for a few seconds.
Water droplets are seen in the inner sides of test tube and the colour of the crystals changes. On continuous heating, a colourless gas X with the smell of burning sulphur is evolved and a residue Y is obtained.
(i) What is the colour of the crystals before and after mild heating?
(ii) Identify gas X and the residue Y formed.
(iii) Write a balanced chemical equation for the above reaction.
(B) Write balanced chemical equations for the following reactions:
(i) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(ii) Sodium metal reacts with water to give sodium hydroxide and hydrogen gas.
Answer: (A) (i) The ferrous sulphate crystals are hydrated crystals (green color). When heated in a boiling tube, the hydrated ferrous crystals turn anhydrous and change their colour to white.
(ii) When heated more strongly, the anhydrous crystals decompose and result in the formation of ferric oxide as residue. The gases obtained are sulphur dioxide and sulphur trioxide.
(iii) The chemical reaction is:
2FeSO4(s) → Fe2SO3(s) + SO2(g)
Ferrous Ferrous Sulphur
sulphate oxide dioxide
+ SO3(g)
Sulphur
rioxide
(B) Balanced chemical equation:
(i) BaCl2(aq) + Al2(SO4)3(aq) →
Barium Aluminium
chloride sulphate
AlCl3(aq) + BaSO4(s)↓
Aluminium Barium
chloride sulphate
(ii) 2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
Sodium Water Sodium Hydrogen
hydroxide
Question: Grapes hanging from the plant do not ferment but after being plucked from the plant can be fermented. Under what conditions do these grapes ferment? Is it a chemical or a physical change?
Answer: When the grapes are attached to the plants, they are still considered as living. They have their immune system intact and thus oxygen reaches in the cell leading to aerobic respiration taking place and no fermentation is possible under aerobic conditions.
After plucking grapes from plants, fermen-tation of sugar is carried out in the presence of yeast, which changes sugar to ethanol and carbon dioxide. This process occurs in the absence of oxygen, i.e., in anaerobic conditions. Here, fermentation is a chemical change as it results in the formation of new substances: alcohol and carbon dioxide.
Question: Lead nitrate solution is added to a test tube containing potassium iodide solution.
(A) Write the name and colour of the compound precipitated.
(B) Write the balanced chemical equation for the reaction involved.
(C) Name the type of this reaction justifying your Answer:
Answer: (A) When lead nitrate solution is added to a test tube containing Potassium iodide solution, a yellow precipitate of lead iodide is produced along with potassium nitrate solution.
(B) Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KO3
Lead iodide (yellow ppt.) Lead Potassium
iodide nitrate
(C) The reaction is double displacement. In this double displacement reaction, two compounds, lead nitrate solution and potassium iodide solution react to form two new compounds, lead iodide and potassium nitrate. An exchange of ions takes place in this reaction.
For example, the lead ions (Pb2+) of lead nitrate react with iodide (I–) of potassium iodide to form a new compound lead iodide (Pb2+I– or PbI2). Similarly, the potassium ions (K+) of Potassium iodide react with the nitrate ions (NO3–) of lead nitrate to form new compound, potassium nitrate (K+NO3– or KNO3). Lead iodide (PbI2) is formed as an insoluble yellow precipitate so it is also called precipitation reaction.
Question: On heating blue coloured powder of copper (II) nitrate in a boiling tube, copper oxide (black), oxygen gas and a brown gas X is formed.
(A) Write a balanced chemical equation of the reaction.
(B) Identify the brown gas X evolved.
(C) Identify the type of reaction.
(D) What could be the pH range of aqueous
solution of the gas X?
Answer: (A) 2Cu(NO3)2 Heat → 2CuO + O2 + 4NO2↑
Copper Copper Oxygen Nitrogen
nitrate oxide dioxide
black) (brown) ‘X’
(B) X is nitrogen dioxide gas (NO2) that has evolved as brown, choking fumes.
(C) A thermal decomposition reaction.
(D) Nitrogen dioxide dissolves in water to form an acidic solution because it is an oxide of non-metal. Therefore, the pH level of this solution is less than 7.
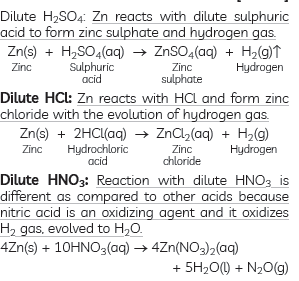
Question:What happens when zinc granules are treated with dilute solution of H2SO4, HCl, HNO3,NaCl and NaOH. Also write their chemical equations, if reaction occurs.
Answer:
Question: Identify the type of reaction taking place in each of the following cases and write the balanced chemical equation for the reactions.
(A) Zinc reacts with silver nitrate to produce zinc nitrate and silver.
(B) Potassium iodide reacts with lead nitrate to produce potassium nitrate and lead iodide.
Answer: (A) When zinc reacts with silver nitrate solution, it forms zinc nitrate and silver.
Zn (s) + 2AgNO3 (aq) → Zn(NO3)2 (aq) + 2Ag(s)
This is a displacement reaction which occurs because zinc is more reactive than silver due to which zinc displaces silver from silver nitrate solution to give to zinc nitrate and silver.
(B) When potassium iodide is added to lead nitrate solution, a yellow precipitate of lead iodide is formed alongwith potassium nitrate solution.
Pb(NO3)2 (aq) + 2KI (aq) → PbI2 (s) + 2KNO3 (aq)
This is a double displacement reaction in which two compounds react by an exchange of ions to form two new compounds. This is also called precipitation reaction as an insoluble solid (precipitate) is formed.
Question: (A) Write the balanced chemical equations for the following reactions:
(i) Zinc + Silver nitrate → Zinc nitrate + Silver
(ii) Aluminium + Copper chloride → Aluminium chloride + Copper
(B) Why is respiration considered an exothermic reaction? Explain.
(C) Why should magensium ribbon be cleared before burning in air?
Answer: (A) Balanced chemical equations for the following reactions:
(i) Zinc + Silver nitrate → Zinc nitrate + Silver
Zn + 2AgNO3 → Zn(NO3)2 + 2Ag
(ii) Aluminium + Copper chloride → Aluminium chloride + Copper
2Al + 3CuCl2 → 2AlCl3 + 3Cu
(B) Respiration is considered as an exothermic reaction because in respiration oxidation of glucose takes place which produces large amount of energy.
This is shown in following chemical equation:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
(C) Magnesium is a reactive metal. When kept exposed in air for a long time, a layer of MgO (magnesium oxide) is formed on the surface of metal. The oxide layer does not burn when flame is brought in contact with metal.
Question: Give characteristic tests for the following gases:
(A) CO2 (B) SO2
(C) O2 (D) H2
Answer: (A) CO2 gas: When CO2 gas is passed through lime water, it forms insoluble calcium carbonate which turns the solution milky.
This is called as lime water test.
Ca(OH)2(aq) + CO2 → CaCO3(s) + H2O
Lime water Carbon Calcium Water
(Colourless) dioxide carbonate(White)
The solution becomes clear in the excess of CO2 because of the formation of soluble
calcium bicarbonate.
CO2 + H2O + CaCO3 → Ca(HCO3)2
Cabon Water Calcium Calcium
dioxide carbonate bicarbonate
(Soluble)
(B) SO2 gas: Due to its acidic nature, sulphur dioxide gas turns moist litmus paper from blue to red.
Sulphur dioxide gas when passed through acidic dichromate solution (orange in colour)
turns it to green because sulphur dioxide is a strong reducing agent.
K2Cr2O7 + 3SO2 + H2SO4 → Cr2(SO4)3
Potassium Chromium
dichromate sulphate
+ K2SO4 + H2O
Chemical Reactions and Equations 25
Sulphur dioxide (SO2) gas when passed through acidic potassium permanganate solution (purple in colour) turns it colourless, because SO2 is a strong reducing agent
2KMnO4 + 2H2O + 5SO2 →
Potasssium Sulphur
permanganate dioxide
(Purple) K2SO4 + 2MnSO4 + 2H2SO4
Potassium Manganese
sulphate sulphate
(Colourless) (Colourless)
(C) O2 gas: The evolution of oxygen (O2) gas during a reaction can be confirmed by bringing a burning candle near the mouth of the test tube containing the reaction mixture. The intensity of the flame increases because oxygen supports burning. Oxygen gas burns brightly with a wooden splinter,which proves the combustible nature of oxygen gas.
C + O2 → CO2 + Heat + Light
Wood Oxygen Carbon
dioxide
(D) H2 gas: Hydrogen (H2) gas burns with a pop sound when a burning candle is brought near it.
Question:What is observed after about 1 hour of adding the strips of copper and aluminium
separately to ferrous sulphate solution filled in two beakers?
Name the reaction if any change in colour is noticed. Also, write chemical equation for the reaction.
Answer: When strip of copper is added to ferrous sulphate solution taken in a beaker, no change is observed.
On adding a strip of aluminium to ferrous sulphate solution taken in another beaker, we observe that the greenish colour of ferrous sulphate solution starts fading and it becomes colourless after about an hour.
Reaction taking place is Displacement reaction.
Equation of the reaction taking place is:
2Al + 3FeSO4 → 3Fe + Al2(SO4)3
Question: A student wants to study a decomposition reaction by taking ferrous sulphate crystals.
Write two precautions he must observe while performing the experiment.
Answer: Precautions to be observed for studying decomposition reaction:
(1) Use only hard boiling test tube.
(2) Hold the test tube in an inclined position away from your body and do not point the mouth of the boiling tube at your neighbours or yourself.
(3) Use a pair of tongs for holding the boiling tube while heating and don’t touch the boiling tube with your bare hands.
(4) Do not inhale the gases emitted directly, it should be inhaled by wafting gently towards your nose.
Question: Answer the following:
(A) Define oxidizing agent.
(B) Translate the following statements into chemical equations and balance them.
(i) Hydrogen gas combines with nitrogen to form ammonia.
(ii) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(iii) Potassium metal reacts with water to give potassium hydroxide and Hydrogen gas.
Answer: (A) Oxidizing agent: It is a substance which loses oxygen or gains hydrogen.
For example in the given reaction:
CuO + H2 → Cu + H2O
CuO has given oxygen, hence it is oxidizing agent.
(B) (i) Hydrogen gas combines with nitrogen to form ammonia.
H2 + N2 → NH3
Balanced:
3H2(g) + N2(g) → 2NH3(g)
(ii) Hydrogen sulphide gas burns in air to give water and sulpur dioxide.
H2S + O2 → H2O + SO2
Balanced:
2H2S(g) + 3O2(g) → 2H2O(l) +
2SO2(g)
(iii) Potassium metal reacts with water to give potassium hydroxide and hydrogen gas.
K + H2O → KOH + H2
Balanced:
2K(s) + 2H2O(l) →2KOH(s) + H2(g)
Question: A student mixes sodium sulphate powder in barium chloride powder.
What change would the student observe on mixing the two powders?
Justify your answer and explain how he can obtain the desired change.
Answer: No reaction takes place when sodium sulphate and barium chloride powders are mixed as no ions are formed in their solid state.
He can get the desired change by taking aqueous solutions of both the reactants as ionic compounds dissociate into ions only in presence of water and then exchange of ions takes place.
The equation for the chemical reaction taking place is:
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl (aq)
Question:While Abhi was about to burn magnesium ribbon in the chemistry laboratory, the teacher asked him to clean the ribbon with a sandpaper before burning it.
What could be the reason for the above instruction by the teacher? After burning the magnesium ribbon, Abhi obtained a white coloured residue. Name this residue. What type of chemical reaction has occurred? Write a balanced chemical equation to explain the reaction.
Answer: When magnesium is kept in the open, the oxygen from the atmosphere reacts with it. This results in the formation of a protective oxide layer on top of magnesium to form magnesium oxide. When the magnesium ribbon is rubbed using sandpaper, the layer of oxide is removed, resulting in exposing the magnesium underneath.
When magnesium ribbon is burnt in air, it results in the formation of magnesium oxide. This is a white coloured substance that is obtained as residue (combination reaction).
2Mg + O2 → 2MgO
Question: A pale green solution of ferrous sulphate was taken in four separate test tubes marked I, II, III and IV. Pieces of Cu, Zn and Al were dropped in test tubes II, III and IV respectively.
In which case(s)
(A) the colour of ferrous sulphate solution will match with the colour in test tube (I)? Give reason.
(B) the colour of ferrous sulphate solution will fade and black mass will be deposited on the surface of the metal?
Answer: (A) In test tube (II) as copper is less reactive than iron, so cannot displace Fe from its salt solution.
(B) In test tubes (III) & (IV) both, because they both, i.e. Zn and Al are more reactive than Fe and will displace Fe from FeSO4.
Question: Write a balanced equation for the following chemical reactions:
(A) Hydrogen + Chloride → Hydrogen chloride
(B) Zinc + Silver nitrate → Zinc nitrate + Silver
Answer: (A) Hydrogen + Chloride → Hydrogen chloride
H2 + Cl2 → 2HCl
(B) Zinc + Silver nitrate → Zinc nitrate + Silver
Zn + 2AgNO3 → Zn(NO3)2 + 2Ag

