Exam Question for Class 10 Science Chapter 3 Metals and Non-Metals
Please refer to below Exam Question for Class 10 Science Chapter 3 Metals and Non-Metals. These questions and answers have been prepared by expert Class 10 Science teachers based on the latest NCERT Book for Class 10 Science and examination guidelines issued by CBSE, NCERT, and KVS. We have provided Class 10 Science exam questions for all chapters in your textbooks. You will be able to easily learn problems and solutions which are expected to come in the upcoming class tests and exams for standard 10th.
Chapter 3 Metals and Non-Metals Class 10 Science Exam Question
All questions and answers provided below for Exam Question Class 10 Science Chapter 3 Metals and Non-Metals are very important and should be revised daily.
Exam Question Class 10 Science Chapter 3 Metals and Non-Metals
Very Short Answer Type Question
Question. Which property makes solder alloy suitable for welding electric wires?
Answer : Its melting point is low which makes solder suitable for welding.
Question. What is the valency of silicon with atomic number 14?
Answer : Si (2,8,4): Its valency is 4 because it can share four electrons to become stable.
Question. What is the valency of phosphorus with atomic number 15?
Answer 😛 (2, 8, 5): Its valency is 3 because it can gain three electrons to become stable.
Question. What is formed when sodium absorb moisture from air? Give equation also.
Answer : Sodium hydroxide and hydrogen gas is formed:
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
Question. Why sulphide and carbonate ores are converted into oxides?
Answer : It is because oxides are more easier to be reduced than sulphides and carbonates.
Question. From amongst the metal, sodium, calcium, aluminium, copper and magnesium, name the metal:
a. which reacts with water only on boiling.
b. another one which does not react even with steam.
Answer :
a. Mg,
b. Cu
Question. Name any one metal which reacts neither with cold water nor with hot water but reacts with heated steam to produce hydrogen gas.
Answer :

Question. A green layer is gradually formed on copper plate when left exposed to air for a week in a bathroom. What could this green substance be?
Answer : CuCO3.Cu(OH)2, Basic copper carbonate.
Question. Why do ionic compounds have high melting point?
Answer : It is due to strong forces of attraction between positively charged and negatively charged ions.
Question. Define metallurgy.
Answer : Metallurgy. All the processes involved in the extraction of metals from its ore is called metallurgy.
Question. What kind of compounds are called Ionic compounds?
Answer : Those compounds which are formed between metals and non-metals by transfer of electrons e.g., NaCl, KCl etc.
Question. Write a balanced chemical equation for the reaction:Aluminium when heated in air. Write the name of the product formed.
Answer : 4Al(s) + 3O2(g) → Heat 2Al2O3(S)
Aluminium oxide
Question. A non-metal X exists in two different forms V and Z.
Y is the hardest known natural substance, whereas Z is a good conductor of electricity. Identify X, Y and Z.
Answer : X is Carbon, Y is Diamond, Z is Graphite.
1. Give one most suitable word for the following statements:
a. Metal oxides which show basic as well as acidic behaviour.
b. Iodine, a shining non-metal.
Answer :
a. Amphoteric oxides.
b. Lustrous.
Question. Write the chemical equation for the reaction taking place when steam is passed over hot Aluminium?
Answer : 2Al(s) + 3H2O(aq) → Heat Al2O3(S) + 3H2(g)
Question. Why does calcium float in water?
Answer : Calcium form hydrogen gas on reaction with water, bubbles of hydrogen gas sticks to the calcium metal and that is why calcium floats in water.
Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g)
Question. Which gas is liberated when a metal reacts with an acid? How will you test this gas?
Answer : Hydrogen gas is formed.
Bring a burning splinter near the gas, it will burn with ‘pop’ sound.
Question. Why do we use copper and aluminium wire for the transmission of electric current?
Answer : It is has 4 to 8 valence electrons, it is a non-metal.
Exception is hydrogen which has 1 valence electron and He which has 2 valence electrons, but these are non- metals.
Question. Name two metals which are found in nature in free state.
Answer : Gold and silver are found in free state.
Question. How are ionic compounds formed?
Answer : Gallium has a melting point 303 K, it melts on palm.
Question. It nature, aluminium is found in combined state whereas silver/gold are found in free state. Give reason.
Answer : Aluminium is reactive metal, therefore it is found in combined state whereas silver/gold are less reactive(noble) metals and so are found in free state.
Question. Why do ionic compounds conduct electricity in molten state and not in solid state?
Answer :Ionic compounds do not conduct electricity in solid state because ions are not free to move. In molten state, ions are free to move.
Question. What is the valency of an element with atomic number 35?
Answer : Br(35) (2, 8, 18, 7): Its valency is equal to 1 because it can gain one electron to become stable.
Short Answer Type Question:
Question: An element E forms an oxide E2O3, which is basic in nature. State whether the element E is a metal or a non-metal?
Answer: As the formula of oxide of element E is E2O3, the valency of E is 3. Since the oxide is basic in nature and we know that metal oxides are basic in nature, E is a metal.
Question: Name two metals that have very low melting points?
Answer: Two metals with very low melting points are gallium and caesium.
Question: Hydrogen gas is not evolved when a metalreacts with nitric acid. Explain.
Answer: Hydrogen gas is not evolved when a metal reacts with nitric acid because nitric acid is a strong oxidising agent. It oxidises the hydrogen produced in water and itself get reduced to any of nitrogen oxides (N2O, NO, NO2). Related Theory ?? Magnesium and manganese react with very dilute
HNO3 to evolve H2 gas.
Question: An element reacts with oxygen to form an oxide which dissolves in dilute hydrochloric acid. The oxide formed also turns a solution of red litmus blue. Is the element a metal or non-metal?
Answer: As the oxide dissolves in dilute hydrochloric acid and also turns a solution of red litmus blue, it is a base. As metal oxides are basic in nature, the given element is a metal.
Question: A metal combines with a non-metal to form a compound Y. Will it dissolve in an organic solvent or not?
Answer: Metals combine with non-metals to form ionic compounds by transfer of electrons. Ionic compounds do not dissolve in organic solvents but are soluble in water.
Question: When calcium metal is added to water, the gas evolved does not catch fire but the same gas evolved on adding potassium metal to water catches fire. Explain why?
Answer: The reaction between potassium and water is a highly exothermic reaction due to which the hydrogen evolved immediately catches fire.
Question: Zinc does not give hydrogen gas on reacting with HNO3. Justify?
Answer: Nitric acid (HNO3) is a strong oxidizing agent which oxidizes the hydrogen produced when zinc reacts with water and itself gets reduced to any of the oxides of nitrogen.
Question: Why do ionic compounds conduct electricity in molten state?
Answer: Ionic compounds conduct electricity in molten state because the ions move freely in molten state since electrostatic force of attraction between oppositely charged ions is overcome due to heat.
Question: An element E combines with O2 to form an oxide E2O, which is a good conductor of electricity. Write the formula of the compound formed when it combines with Chlorine?
Answer: As the formula of oxide of E is E2O and E is a good conductor of electricity, E is a metal with valency 1. The formula of its compound with chlorine will be ECl.
Question: Why should we not throw small pieces of sodium into a sink in the laboratory?
Answer: Metals like sodium and potassium react very violently with cold water and the reaction is so exothermic that the hydrogen evolved may even catch fire. Therefore, small pieces of sodium are not thrown into a sink in the laboratory.
Short Answer Type Question:
Question: A shining metal ‘M‘, on burning gives a dazzling white flame and changes to a white powder ‘N‘.
(A) Identify ‘M‘ and ‘N‘.
(B) Represent the above reaction in the form of a balanced chemical equation.
(C) Does ‘M‘ undergo oxidation or reduction in this reaction? Justify.
Answer: (A) M—Magnesium
N—Magnesium oxide.
(B) 2Mg(s) + O2(g) → 2MgO(s)
magnesium oxygen magnesium oxide
Or
2M + O2 → 2MO2
(C) ‘M‘ metal will undergo oxidation reaction as oxygen is added to metal ‘M‘ and MO2 (metal oxide) is formed.
Explanation: When a piece of shining metal ‘M‘ is burnt in air, a white powder of metal oxide is formed. Shining metal is magnesium ribbon which burns with a dazzling white flame and white powder is formed which is magnesium oxide.
Question: When a metal X is treated with cold water, it gives a basic salt Y with molecular formula XOH (molecular mass = 40) and liberates a gas Z which easily catches fire. Identify X, Y and Z and also write the reaction involved.
Answer: X is Na, Y is NaOH and Z is H2. The reaction is:
2Na + 2H2O → 2NaOH + H2 + Heat energy It is given that the molecular formula of Y = XOH and molecular mass = 40.
Let the atomic weight of metal X be a.
Then, molecular mass of XOH = a + 1?? + 1 = 40.
Then, a = 40 – 1?? = 23.
The atomic weight of sodium is 23, so metal X is sodium (Na).
Sodium reacts with water to give hydrogen gas (Z), that catches fire.
2Na + 2H2O(Cold) → 2NaOH + H2↑
So, metal X is sodium (Na), Y is (sodium hydroxide) and Z is H2 (hydrogen gas).
Question: A non-metal X exists in two different forms Y and Z. Y is the hardest natural substance, whereas Z is a good conductor of electricity. Identify X, Y and Z.
Answer: Here, X is carbon; Y is diamond and Z is graphite.
Non-metal X is carbon (C).
Carbon exists in different forms. These different forms of carbon are called the allotropes of carbon. Diamond and graphite are allotropes of carbon.
Y is diamond because diamond is the hardest natural substance.
Z is graphite as it is a good conductor of electricity due to the presence of free mobile electrons.
Question: Write the balanced chemical equation for the following reactions and identify the type of reaction in each case:
(A) In Thermite reaction, iron (III) oxide reacts with aluminium and gives molten iron and aluminium oxide.
(B) Magnesium ribbon is burnt in an atmosphere of nitrogen gas to form solid magnesium nitride.
(C) Chlorine gas is passed in an aqueous potassium iodide solution to form potassium chloride solution and solid iodine.
Answer: (A) Thermite reaction, iron (III) oxide reacts with aluminium and gives molten iron and aluminium oxide.
Fe2O3(s) + 2Al(s) → 2Fe(l) + Al2O3(s)
Iron (III) oxide Iron Aluminium oxide
+ Heat + Light
It is an exothermic redox reaction as well as displacement reaction because aluminium displaces iron from iron (III) oxide.
Explanation:

Since at is more reactive than Fe so it displaces. Fe from its oxide. Both oxidation and reduction are taking place at the same two so it is redox reaction.
(B) Magnesium ribbon is burnt in an atmosphere of nitrogen gas to form solid magnesium nitride.
3Mg(s) + N2(g) → 2Mg3N2(s)
It is a combination reaction as well as redox reaction.
(C) Chlorine gas is passed in an aqueous potassium iodide solution to form potassium chloride solution and solid iodine.
2KI(aq) + Cl2(g) → 2KCl(aq) + I2(s)
It is a displacement as well as redox reaction.
Question: Metal A, which is used in thermite process, when heated with oxygen, gives an oxide B, which is amphoteric in nature. Identify A and B. Write down the reactions of oxide B with HCl and NaOH.
Answer: The metal A is aluminium and B is Al2O3/ 4Al(s) + 3O2(g) → 2Al2O3(s)
Reactions of aluminium oxide with HCl and NaOH are as given below:
Al2O3(s) +6HCl(aq) → 2AlCl3(aq) + 3H2O(l)
Al2O3(s) + 2NaOH(aq) → 2NaAlO2(aq) + H2O(l)
Metal A is aluminium (Al) that is used in the thermite process. Al reacts with oxygen to form aluminium oxide [Al2O3(B)], which is amphoteric in nature. Thus, aluminium oxide can exhibit acidic as well as basic nature.
Hence, A = aluminium and B = aluminium oxide
Question: Compare in tabular form the reactivities of the following metals with cold and hot water:
(A) Sodium (B) Calcium (C) Magnesium
Answer:
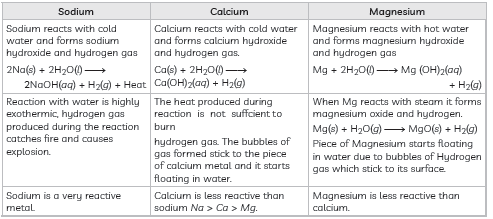
Question: What are ionic compounds? Why do ionic compounds not conduct electricity in the solid state?
Answer: Ionic compounds are the compounds formed by the combination of oppositely charged ions formed by transfer of electrons from one atom to another.
Ionic compounds do not conduct electricity in solid state as they have a rigid structure as ions are not free to move due to strong electrostatic forces of attraction between them.
Question: Give the characteristic tests for the following gases:
(A) CO2 (B) SO2 (C) H2
Answer: Characteristic tests for the following gases:
(A) CO2 gas: When CO2 gas is passed through lime water; it forms insoluble calcium carbonate which turns the solution milky.
This is called as lime water test.
Ca(OH)2 + CO2 → CaCO3 + H2O
The solution becomes clear in excess of CO2 because of formation of soluble calcium bicarbonate.
CO2 + H2O + CaCO3 → Ca(HCO3)2
(B) SO2 gas: Due to acidic nature, sulphur dioxide gas turns moist litmus paper from blue to red. It also changes the orange colour of acidified potassium dichromate solution to green.
K2Cr2O7(aq) + 3SO2(g) + H2SO4(aq) →
Cr2(SO4)3(aq) + K2SO4(aq) + H2O(l)
(C) H2 gas: In the presence of atmospheric oxygen, hydrogen gas burns with a pop sound.
Question: Give the formulae of the stable binary compounds that would be formed by the combination of following pairs of elements:
(A) Mg and N2 (B) Li and O2
(C) Al and Cl2 (D) K and O2
Answer: (A) Mg and N2 Mg3N2 (Magnesium nitride)
(B) Li and O2 Li2O (Lithium oxide)
(C) Al and Cl2 AlCI3 (Aluminium chloride)
(D) K and O2 K2O (Potassium oxide)
Question: An alkali metal A gives a compound B (molecular mass = 40) on reacting with water.
The compound B gives a soluble compound C on treatment with aluminium oxide. Identify A, B and C and give the reactions involved.
Answer: A is Na, B is NaOH and C is NaAlO2
Reactions involved are:
2Na + 2H2O → 2NaOH + H2
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Let the atomic weight of alkali metal A be a.
When it reacts with water, it forms A compound B having molecular mass 40.
Therefore, a + 16+ 1 = 40
a = 40 –17 = 23
We know that the atomic weight of Na (sodium) is 23.
Therefore, the alkali metal (A) is Na. Sodium reacts with water to form sodium hydroxide. So, compound B is sodium hydroxide (NaOH).
2Na + 2H2O → 2NaOH + H2
Sodium hydroxide reacts with aluminium oxide (Al2O3) to give sodium aluminate (NaAlO2).
Thus, C is sodium aluminate (NaAlO2).
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Question: Answer the following:
(A) Write the electron-dot structures for sodium, oxygen and magnesium.
(B) Show the formation of Na2O and MgO by the transfer of electrons.
(C) What are the ions present in these compounds?
Answer: The electronic dot structure for sodium, oxygen and magnesium is shown below:

(B) Formation of Sodium oxide Na2O:
Atomic number of sodium (Na) = 11
Its electronic configuration = 2, 8, 1
Atomic number of oxygen (O) = 8
Its electronic configuration = 2, 6
Each sodium atoms can lose only one electron and attains stable configuration like that of Neon (2, 8)
Na → Na+ + e–
Sodium ion
But each oxygen atom requires two electrons to attain stable configuration of neon (2, 8) so two sodium atoms.
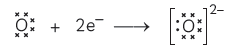
So two atoms will lose two electrons (i.e. one each)

Formation of Magnesium oxide:
Atomic number of magnesium (Mg) = 12
Electronic configuration = 2, 8, 2
It loses two electrons from its valence shell and acquires electronic configuration of neon

Atomic number of oxygen = 8
Its electronic configuration = 2, 6
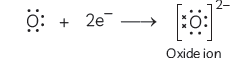
It gains two electrons to acquire the stable configuration of neon (2, 8) and becomes oxide ion (O2–)
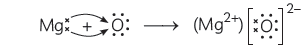
(C) The ions present in sodium oxide compound (Na2O) are sodium ions (2Na+) and oxide ions (O2–).
Ions present in Magnesium oxide compound (MgO) are magnesium ions Mg2+ and oxide ions (O2–).
Question: A metal M does not liberate hydrogen from acids but reacts with oxygen to give a black coloured product. Identify M and the black coloured product and also explain the reaction of M with oxygen.
Answer: The metal M is Cu and the black coloured product is copper oxide (CuO).
Because of less reactivity, copper metal does not release hydrogen gas with acid.
Cu(s) + HCl → No reaction
Copper reacts with oxygen to form copper oxide. The reaction of metal M with oxygen is as follows:
2Cu + O2 → 2CuO
Hence, metal M is copper.
Question: Name one metal and one non-metal that exist in liquid state at room temperature. Also name two metals having melting point less than 310 K (37°C).
Answer: (1) Metal that exists in liquid state at room temperature is mercury.
(2) Non-metal that exists in liquid state at room temperature is bromine.
(3) Metals having melting point less than 310 K are cesium (Cs) and gallium (Ga).
Question: When a metal X is treated with cold water, it gives a basic salt Y with molecular formula XOH (molecular mass = 40) and liberates a gas Z which easily catches fire. Identify X, Y and Z and also write the reaction involved.
Answer: Given, molecular formula of Y = XOH and molecular mass of Y = 40
So, atomic mass of metal X would be, M + 16+ 1 = 40
M = 40 – 17 = 23
Metal with atomic mass 23 is sodium(Na) and reaction of it with cold water will form the base sodium hydroxide (NaOH) and liberates H2 gas which easily catches fire.
2Na + 2H2O(Cold) → 2NaOH + H2
X = Sodium (Na)
Y = Sodium hydroxide (NaOH)
Z = Hydrogen gas (H2)
Question: Answer the following questions:
(A) Predict which of the following elements will form cation and which will form anions?
(a) Na (b) Al
(c) Cl (d) O
(B) Also name two elements that are inert in nature.
Answer: If sodium (Na) loses 1 electron (the electron) from M shell, then its outermost shell will be L having 8 electrons and has a stable octet.
The nucleus of this atom has 11 protons but number of electrons has become 10 so there is a net positive charge and becomes Na+ cation similarly in Al.
Al → Al3+ + 3e–
2, 8, 3
Since Al loses 3 electrons and becomes Al3+ cation.
Elements which will form cations: Na and Al (Sodium and Aluminium) Elements which will form anions: Cl and O (Chlorine and Oxygen)
(B) Cl atom has 7valence electrons and it needs 1 electron to acquire stable octet in M shell.
After it gains one electrons, the number of protons will remain 17 but number of electrons become 18 so then chlorine atom gets a unit negative charge and becomes
Cl– anion
Cl + e– → Cl
2, 8, ??
Similarly oxygen atom acquires 2 electrons as it values shell as ?? electrons. The number of protons will remain 8 but number of electrons becomes 10 so giving it a negative charge O2– anion
O + 2e– → O2–
2, 6
He and Ne (Helium and Neon) are two inert elements.
The outermost shell in helium is K with 2 electrons and in Ne, the outermost shell L has 8 electrons. It means that both the elements have stable octet. They cannot lose or gain electrons and are unreactive.
So, we can say these two are two inert elements.
Question: An element forms an oxide A2O3 which is acidic in nature. Identify A as a metal or non- metal.
Answer: Metals usually form basic oxides like BaO, MgO, Na2O etc. Unlike metals, non-metals tend to form acidic oxides. Since an oxide of element is acidic in nature, therefore, A will be a non-metal.
The formula for oxide is A2O3, which means the charge on the element must be +3 or the element should have 3 valence electrons.
This implies that the element is boron and the formula for oxide would be B2O3.
Question: A metal M forms an oxide having the formula M2O3. It is dissolved both in dilute sulphuric and dilute sodium hydroxide solution. Identify the metal and write equations for the reaction involved. What criteria would you use to assess it?
Answer: Here the metal oxide dissolves in sulphuric as well as sodium hydroxide solution. That means metal oxide is showing acidic as well as basic character. So metal oxide is amphoteric oxide.
The metal is Aluminium (Al).
It forms aluminium oxide (Al2O 3) which is amphoteric that it shows the nature of both acidic and basic.
Al2O3 + 3H2SO4 → Al2(SO4)3 + 3H2O
Salt
Al2O3 + 6NaOH → 2NaAlO2 + H2O
Salt
Question: Rohan observed that if a small piece of sodium is added to water, it catches fire, whereas a piece of calcium added to the water does not catch fire. Can you explain his observations with the help of the chemical equations?
Answer: Sodium reacts vigorously with cold water, forming sodium hydroxide and hydrogen gas.
The chemical reaction is as follows:
2Na + 2H2O → 2NaOH + H2(g)↑ + Heat
Sodium Water Sodium Hydrogen
hydroxide
The reaction of sodium metal with water releases a large amount of heat, due to which the hydrogen gas formed during the reaction catches fire easily. Thus, sodium is a very reactive metal.
On the other hand, calcium reacts with cold water to form calcium hydroxide and hydrogen gas. The chemical reaction is as follows:
Ca + 2H2O → Ca(OH)2↓ + H2
Calcium Water Calcium Hydrogen
hydroxide
The heat produced in this reaction is less. It is not sufficienttoburnthehydrogengasformed. The reaction of calcium metal with water is less violent as compared to sodium. So, we can say that calcium is less reactive than sodium.
Question: When most of the metals are treated with nitric acid, they do not produce hydrogen gas. Why?
Answer: Nitric acid is a strong oxidising agent. When hydrogen gas is formed in a reaction between a metal and dilute nitric acid, the nitric acid oxidises this hydrogen to form water. So, in reactions between metals with dilute nitric acid, no hydrogen gas is evolved.
Question: A solution of CuSO4 was kept in an iron pot.
After a few days, the iron pot was found to have a number of holes in it. Explain the reason in terms of reactivity. Write the equation of the reaction involved.
Answer: In reactivity series, iron is placed above copper.
So, we can say that iron is more reactive as compared to copper and it can displace copper from its compounds.
When copper sulphate solution is placed in an iron pot, iron reacts with copper sulphate and forms iron sulphate with copper.
Fe + CuSO4 → FeSO4 + Cu
So, the blue colour of copper sulphate solution fades to light green due to the formation of iron sulphate and holes are produced at places where iron metal has reacted.
Long Answer Type Question
Question: (A) Explain the formation of ionic compound, Al2O3 with electron-dot structure:
(Given: Atomic no. of Al and O are 13 and 8 respectively)
(B) What happens when (Report only observations)
(a) a reactive metal reacts with a dilute mineral acid?
(b) an amphoteric oxide reacts with sodium hydroxide solution?
(c) a metal of low reactivity is dropped in the salt solution of a metal of high reactivity?
(d) a metal carbonate is treated with acid?
Answer: (A) Formation of Ionic compound Al2O3
Atomic number of Al —13
Atomic number of O — 8 Electronic configuration of Al = 2, 8, 3
Electronic configuration of O = 2,6
All loses 3 electrons from the valence shell to acquire the nearest noble gas (Neon) configuration and form Al3+
Al → Al3+ + 3e–
2, 8, 3 2, 8
Oxygen gains only 2 electrons in the valence shell to acquire the nearest noble gas (Neon) configuration and form O2–
O + 2e– → O2–
2,6 2, 8
Al has to lose 3 electrons and oxygen atom can only 2 electrons, therefore 2 atoms of Al and 3 atoms of oxygen are required :

(B) (i) When a reactive metal reacts with dilute mineral acid Observations:
(i) The reaction will be violent.
(ii) Evolution of hydrogen gas is seen as bubbles of gas forming in the reaction mixture.
Explanation:
2Na(s) + 2HCl(aq) → 2NaCl(aq) + H2(O)
Reactive Mineral Metal Hydrogen
Metal acid chloride gas
(ii) An amphoteric oxide reacts with sodium hydroxide solution and forms salt and water. Eq.
Al2O3(s) + NaOH(aq) → NaAlO2(aq) + H2O(l)
Aluminium Sodium Sodium water
oxide hydroxide aluminate
(Amphoteric (Base) (Salt)
ZnO + NaOH → Na2ZnO2(aq) + H2O(l)
(iii) A metal of low reactivity is dropped in the salt solution of a metal of high reactivity.
No reaction will take place as a more reactive metal displaces a less reactive metal from its salt solution.
(iv) When a metal carbonate is treated with acid, brisk effervescence of carbon dioxide is given off alongwith formation of salt.
Eq.
Na2CO3(s) + 2HCl(aq) → 2NaCl(aq) + CO2(g) + H2O(l)
Question: (A) (i) Write two properties of gold which make it the most suitable metal for ornaments.
(ii) Name two metals which are the best conductors of heat.
(iii) Name two metals which melt when you keep them on your palm.
(B) Explain the formation of ionic compound CaO with electron-dot structure. Atomic
numbers of calcium and oxygen are 20 and 8 respectively.
Answer: (A) (i) Two properties of gold which makes it the most suitable metal for ornaments are:
(a) Gold is an unreactive metal and therefore does not lose its shine easily.
(b) Gold is a highly malleable metal.
(c) Gold is the most ductile metal which makes it the best choice for making ornaments.
(ii) The two best conductors of heat are silver and copper.
(iii) Gallium and caesium are two metals which melt if kept in our palms as they have very low melting points.
(B) Atomic number of calcium = 20
Electronic configuration = (2, 8, 8, 2)
Atomic number of oxygen = 8
Electronic configuration = (2,6)
Calcium atom loses 2 valence electrons to form Ca2+ ion and oxygen atom gains 2 electrons to form O2– ion.
This way both attain their nearest inert gas configuration. The formation of ionic compound CaO is shown below; Calcium atom oxygen atom calcium ion oxide ion.
Question: Explain the following:
Reactivity of Al decreases if it is dipped in HNO3.
Answer: When aluminium (Al) is dipped in nitric acid (HNO3), a layer of aluminium oxide (Al2O3) is formed over the metal surface. This happens because nitric acid is a strong oxidising agent.
The layer of aluminium oxide prevents further reaction of aluminium. This is the reason why the reactivity of aluminium decreases.
Question: (A) Complete and balance the following chemical equations:
(i) Al2O3 + HCl →
(ii) K2O + H2O →
(iii) Fe + H2O →
(B) An element ‘X‘ displaces iron from the aqueous solution of iron sulphate. List your observations if the element ‘X‘ is treated with the aqueous solutions of copper sulphate, zinc sulphate and silver nitrate.
Based on the observations arrange X, Zn, Cu and Ag in increasing order of their reactivities.
Answer: (A) (i) Al2O2(s) + 6HCl(l) → 2AlCl3(aq)(l) + 3H2O(l)
(Aluminium (Hydrochloric Aluminium chloride water
oxide) acid)
(ii) K2O + H2O(l) → 2KOH(aq) + 4H2(g)
(Pot. (Water) Potassium
oxide) hydroxide
(iii) 3Fe(s) + 4H2O(g) → Fe3O4(s) + 4H2(g)
Iron (steam) Iron (II, III) oxide (Hydrogen)
(B) An element ‘X‘ displaces iron from the aqueous solution of iron sulphate. A more
reactive metal displaces a less reactive metal from its salt solution. It means element ‘X‘ is more reactive than iron.
X + FeSO4(aq) → XSO4(aq) + Fe
The pale green colour of FeSO4 will fade away slowly when element ‘X‘ is treated with the aqueous solutions of CUSO4.
X + CuSO4(aq) → XSO4(aq) + Cu
Copper is less reactive than iron, iron is less reactive than X. It means copper is less reactive than X.
The blue colour of CuSO4 will fade away slowly.
The deposits of metal ‘X‘ will be seen on the copper strip.
X will be able to displace sliver from its silver nitrate solution as silver is less reactive than copper even.
The increasing order of reactivities of these metals X, Zn, Cu and Ag is as :
Ag < Cu < Zn < X
Explanation:
(i) X + FeSO4(aq) → XSO4(aq) + Fe
(Pale green) (Colourless)
(ii) X + CuSO4(aq) → XSO4(aq) + Cu
(Blue) (Colourless)
(iii) X + ZnSO4(aq) → XSO4(aq) +Zn
(iv) X + AgNO3(aq) → XNO3(q) + Ag
(Colourless) (Colourless)
X displaces iron, iron is more reactive that copper and silver that is why X is highly reactive metal of all given metals.
Question: Of the three metals X, Y and Z, X reacts with cold water, Y with hot water and Z with steam only. Identify X, Y and Z and also arrange them in increasing order of reactivity.
Answer: X is alkali metal, Na or K.
Y is alkaline earth metal, Mg or Ca.
Z is Fe.
X reacts with cold water, so it must be very reactive like alkali metals, like sodium. Sodium reacts with water to form sodium hydroxide and hydrogen gas.
2Na + 2H2O → 2NaOH + H2
Y metal can react with hot water, so it must be a little less reactive than X i.e., alkaline Earth metal. So, Y can be magnesium (Mg) which reacts with hot water to form magnesium hydroxide.
Mg + 2H2O → Mg(OH)2 + H2
Z metal which reacts with steam must be iron that forms iron (III) oxide with steam.
3Fe + 4H2O → Fe3O4 + 4H2
Hence, the increasing order of reactivity of the given metals is:
Z (Fe) < Y (Mg) < X (Na)
Question: Explain the following:
(A) Sodium chloride is an ionic compound which does not conduct electricity in solid state, whereas it does conduct electricity in molten state as well as in aqueous solution.
(B) Reactivity of aluminium decreases if it is dipped in nitric acid.
(C) Metals like calcium and magnesium are never found in their free state in nature
Answer: (A) We know that solid sodium chloride is made up of ions but it does not conduct electricity. This is because of the fact that the sodium ions and chloride ions are held together in fixed positions in the sodium chloride crystal. They cannot move freely.
When sodium chloride is dissolved in water to make aqueous solution, it becomes a good conductor of electricity. On dissolving in water, the sodium chloride crystal breaks, sodium ions (Na+) and chloride ions (Cl–) become free to move and as a result conduct electricity.
(B) A layer of aluminium oxide is formed on the metal when aluminium is dipped in nitric acid. This happens because nitric acid is a strong oxidizing agent. The layer of aluminium oxide acts as a barrier to prevent further reaction of aluminium. As a result, the reactivity of aluminium decreases.
(C) Metals like calcium and magnesium are never found in free state in nature because these metals are very reactive and readily combine with other elements to form a compound.
Question: List in tabular from three chemical properties
on the basis of which we can differentiate
between a metal and a non-metal.
Answer: Chemical properties used to differentiate between metal and non-metals:

Question: (A) What are amphoteric oxides? Choose the amphoteric oxides from amongst the following oxides: Na2O, ZnO, Al2O3, CO2,H2O.
(B) Why is it that non-metals do not displace hydrogen from dilute acids?
Answer: (A) Those metal oxides which show basic as well as acidic behaviour are known as amphoteric oxides. Amphoteric oxides react with both acids as well as bases to form salts and water. Al2O3, ZnO are amphoteric oxides among the given oxides.
(B) Non-metals do not displace hydrogen from dilute acids because in order to displace hydrogen ions (H+) of an acid and convert them into hydrogen gas, electrons should be supplied to the hydrogen ions (H+) of the acid.
A non-metal is itself an acceptor of electrons. Hence it cannot give electrons to the hydrogen ions of the acid to reduce them to hydrogen gas. As a result, non- metals are not able to displace hydrogen ions from acids to form hydrogen gas.
Question: Out of three metals P, Q and R, P is less reactive than Q and R is more reactive than P and Q both. Suggest an activity to arrange P, Q and R in order of their decreasing reactivity.
Answer: (A) Take salt solutions, say sulphate solutions, of metals P and Q in separate test tubes.
(B) Put a strip of metal R in both the test tubes.
(C) The solution becomes colourless in both the test tubes which shows that metal R displaces P and Q ions from their solutions.
(D) Next, put a strip of metal P in the test tube containing salt solution of metal Q.
(E) No reaction takes place as P cannot displace Q ions from its solution.
The decreasing order of reactivity of given metals is : R > Q > P
Question: Name the following:
(A) Metal that can be cut by knife
(B) Lustrous non-metal
(C) Metal that exists in liquid state at room temperature
(D) Most malleable and ductile metal
(E) Metal that is best conductor of electricity
(F) Non-metal that can exist in different forms
Answer: (A) Metal which can be cut by knife—Sodium
(B) Lustrous non—metal—Iodine
(C) Metal that exists in liquid state at room temperature—mercury
(D) Most Malleable and ductile metal—Gold
(E) Metal that is best conductor of electricity— Silver
(F) Non-metal that can exist in different forms—Carbon
Question:(A) When calcium metal is added to water, the gas evolved does not catch fire but the same gas evolved on adding potassium metal to water catches fire. Explain.
(B) Name a metal for each case:
(i) It displaces hydrogen gas from nitric acid.
(ii) It does not react with any physical state of water.
(iii) It does not react with cold as well as hot water but reacts with steam.
Answer: (A) More heat is evolved during the reaction of potassium metal with water due to which the hydrogen gas formed catches fire. On the other hand, less heat is evolved during the reaction of calcium metal with water, which cannot make the hydrogen gas burn.
(B) (i) Zinc because it is more reactive than hydrogen and can easily displace hydrogen from its compounds like water and acids to form hydrogen gas.
(ii) Copper because it is a highly unreactive metal.
(iii) Iron because it is a very less reactive metal.
Question: State five uses each of metals and non- metals.
Answer: Uses of metals:
(A) Lead metal is used in making car batteries.
(B) Zinc is used for galvanizing iron to protect it from rusting.
(C) Iron, copper and aluminium are used to make utensils.
(D) Copper and aluminium metals are used to make electrical wires.
(E) Aluminium is used to make aluminium foil for packaging materials.
Uses of non-metals:
(A) Hydrogen is used in the hydrogenation of vegetable oils.
(B) Carbon is used to make electrodes of electrolytic cells and dry cells.
(C) Nitrogen is used in the manufacture of ammonia, nitric acid and fertilizers.
(D) Sulphur is used for producing sulphuric acid.
(E) Liquid hydrogen is used as rocket fuel.

