Exam Question for Class 10 Science Chapter 4 Carbon and Its Compound
Please refer to below Exam Question for Class 10 Science Chapter 4 Carbon and Its Compound. These questions and answers have been prepared by expert Class 10 Science teachers based on the latest NCERT Book for Class 10 Science and examination guidelines issued by CBSE, NCERT, and KVS. We have provided Class 10 Science exam questions for all chapters in your textbooks. You will be able to easily learn problems and solutions which are expected to come in the upcoming class tests and exams for standard 10th.
Chapter 4 Carbon and Its Compound Class 10 Science Exam Question
All questions and answers provided below for Exam Question Class 10 Science Chapter 4 carbon and compound are very important and should be revised daily.
Exam Question Class 10 Science Chapter 4 Carbon and Its Compound
Very Short Answer Type Questions:
Question: Covalent compounds are generally poor conductors of electricity. Why?
Answer: Covalent compounds are poor conductors of electricity because these compounds do not have any charged particles as they are made by sharing of electrons.
Explanation: The conduction of electricity through a solution involves the the movement of charged particles/ ions. Covalent compounds do not contain ions and hence are generally bad conductors of electricity.
Question. Name the following compound:
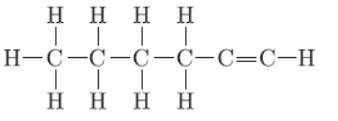
Answer: 1-Hexyne
Question. Write the molecular formula of 2nd and 3rd member of the homologous series whose first member is ethyne.
Answer:
(1) HC / C—CH3 Propyne
(2) HC / C—CH2—CH3 1-Butyne
Question. Write the molecular formula of 2nd and 3rd member of the homologous series whose first member is ethene.
Answer:
(1) CH2=CH—CH3 Propene
(2) CH2=CH—CH2—CH3 1-Butene
Question. Write the next homologue of each of the following:
a. C2H4
b. C4H6
Answer:
(1) C3H6, CH2=CH—CH3
(2) C5H8, HC=C—CH2—CH2—CH3
Question. Name the following compounds:
a. CH3 CH2 OH
O
II
b.CH3 -C- H
Answer:
a. Ethanol
b. Ethanal
Question. Select the saturated hydrocarbons from the following:
C3H6, C5H10, C4H10, C6H14, C2H4
Answer: C4H10, C6H14 are saturated hydrocarbons
Question. Write the name and structure of an alcohol with three carbon atoms in its molecules.
Answer: CH3CH2CH2OH, 1-Propanol
Question. Write the molecular formula of first two members of homologous series having functional group —Cl.
Answer:
(1) CH3Cl Chloromethane
(2) CH3CH2Cl Chloroethane
Question. Write the name and structure of an alcohol with four carbon atoms in its molecule.
Answer: CH3CH2CH2CH2OH, 1-Butanol
Question. Write the name and structure of an aldehyde with four carbon atoms in its molecule.
Answer:
O
II
CH3 – CH2 – CH2 – C – H Butanal
Question. Which element exhibits the property of catenation to maximum extent and why?
Answer: Carbon because it can form strong covalent bond with other carbon atoms due to smaller size.
Question. Unsaturated hydrocarbon gives a yellow flame with lot of black smoke when burnt in oxygen. Give reason.
Answer: Unsaturated hydrocarbons have more amount of carbon, therefore burns with smoky flame due to incomplete combustion.
Question. Name the process by which unsaturated fats are changed into saturated fats.
Answer: Hydrogenation
Question. Write the name of each of the following functional groups:
a. —OH b. – C –
II
O
Answer:
a. Alcohol
b. Ketone
Question. Write the name and molecular formula of the first member of homologous series of alkynes.
Answer: HC=CH, Ethyne
Question. Write the name and formula of fourth member of alkane series.
Answer: CH3—CH2—CH2—CH3, Butane
Question. What is a homologous series of carbon compounds?
Answer: Homologous series is series of organic compounds which have same functional group and similar chemical properties and each successive member has more —
CH2 unit than the previous one.
Question. What will you observe on adding a 5% alkaline KMnO4 solution drop by drop to some warm ethanol taken in a test tube? Write the name of the compound formed during the above chemical reaction.
Answer: The purple colour of KMnO4 decolourises and ethanoic acid will be formed
C2H5OH Alk KMNO4 → CH2OOH
ethanol ethanoic acid
Question. Butanone has four carbon per molecule of a compound.
Name the functional group present in it.
Answer: Ketone
Question. A colourless gas X has a formula C3H6. It decolourises bromine water. Write the chemical formula of ‘X’.
Answer: CH2=CH—CH3, Propene
Question: Write the name and formula of a carbon compound having – OH functional group.Answer: Name and formula of a carbon compound having – OH functional group:

Question: What is a saturated hydrocarbon? Write the formula of any one saturated hydrocarbon. Answer: A saturated hydrocarbon is a hydrocarbon in which the carbon atoms have only single covalent bonds between them.
Formula of any one saturated hydrocarbon is
CH4, C2H6, C3H8, C4H10, C5H12
Question: Write the molecular formula of the first two members of the homologous series having functional group —COOH.
Answer: The molecular formula of the first two members of the homologous series having functional group —COOH is HCOOH and CH3COOH
Question: Write the molecular formula of (i) Methane and (ii) Ethanol.
Answer: The molecular formula of (i) Methane: CH4
(ii) Ethanol: C2H5OH
Question: Why does carbon become stable after sharing four electrons? What type of bond is
formed by sharing?
Answer: The element carbon has atomic number as ?? and its electronic configuration is 2,4 and it is tetravalent . Thus, carbon has 4 valence electrons. It can neither gain nor lose 4 electron to acquire the nearest noble gas configuration.
Only way is to share the four valence electrons with the electrons of other atoms The type of bond formed by sharing of electrons is covalent bond.
Question: Write the next homologue of each of the following:
(A) C2H4 (B) C4C6
Answer: (A) C3H6
(B) C5H8
Question: Define catenation.
Answer: The property of self-linking of atoms of an element through covalent bonds in order to form straight chain, branched chains or cyclic chains of different sizes is called catenation.
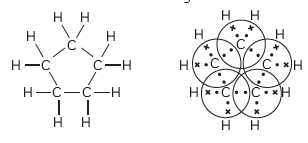
Question: What will be the formula and electron dot structure of cyclopentane?
Answer: The formula for cyclopentane is C5H10. Its electron dot structure is given below:
Question. An alkene ‘P’ has three carbon atoms and an alcohol ‘Q’ has four carbon atoms. Write the formulae of P and Q.
Answer. ‘P’ is CH3–CH=CH2, ‘Q’ is CH3CH2CH2CH2OH
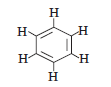
Question. Write the molecular formula of benzene and state the number of double bonds in its structure.
Answer.C6H6, It has three double bonds
Question.What is homologous series?
Answer.It is a series of organic compounds having same functional group and similar chemical properties.
Question. The molecular formula of two members of a homologous series are C3H4 and C6H10. Write the molecular formula of a member of this family with five carbon atoms in a molecule.
Answer.C5H8
Question. Write the general formula of alkenes. Write the name of the simplest alkene.
Answer.CnH2n, Ethene is simplest alkene.
Question. Write the next homologue of each of the following: (i) C2H4, (ii) C4H6
Answer.(i) C3H6, (ii) C5H8
Question. Write the structure of an alcohol with three carbon atoms in the molecule.
Answer.
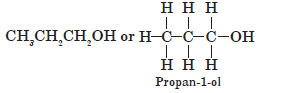
Question. Write the molecular formula of alcohol derived from butane.
Answer.C4H9OH or CH3CH2CH2CH2OH (Butan-1-ol)
Question. Write the molecular formula of an alkyne containing 10 atoms of hydrogen.
Answer.C6H10
Question. Write the name and molecular formula of the fourth member of alkane series.
Answer.C4H10, Butane (CH3CH2CH2CH3)
Question. Write the name and formula of second member of homologous series with general formula CnH2n+2
Answer.C2H6, Ethane
Question. Write the name and formula of second member of homologous series having general formula CnH2n–2.
Answer.C3H4, Propyne
Question. Which of the following organic compounds belong to the same homologous series:
C2H6, C2H6O, C2H6O2, CH4O
Answer.C2H6O (C2H5OH) and CH4O (CH3OH)
Question. The formula of citric acid is shown below:
State the name of —COOH functional group in citric acid.
Answer.Carboxylic acid
Question. The molecular formula of ‘A’ is C10H18 and ‘B’ is C18H36. Name the homologous series to which they belong.
Answer.‘A’ belongs to Alkynes, ‘B’ belongs to Alkenes.
Question. Write the next homologous of CH3CH2OH and HCOOH.
Answer.CH3 – CH2 – CH2OH. Propanol and, CH3COOH ethanoic acid
Question. Write the molecular formula of first two members of homologous series having functional group Cl.
Answer.The general formula of the compounds having – Cl functional group is CnH2n + 1Cl. Its two members are:
(i) CH3Cl (ii) CH3 – CH2 – Cl
Question. Write the molecular formula of the 2nd and the 3rd member of the homologous series whose first member is methane (CH4).
Answer.
(i) CH3CH3 (Ethane); where n is 2
(ii) CH3CH2CH3 (Propane); where n is 3
Question. How many covalent bonds are there in a molecule of ethane (C2H6)?
Answer.There are 7 covalent bonds.
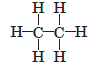
Question. Write the electron dot diagram of ethane (C2H6) molecule.
Answer.

Question. Write the number of covalent bonds in propane, C3H8.
Answer.There are 7 covalent bonds.

Question. Write the number of covalent bonds in the molecule of butane (C4H10).
Answer.There are 13 covalent bonds.

Question. State the valency of the carbon atom in (i) an alkane (ii) an alkyne.
Answer.(i) Four, (ii) Four
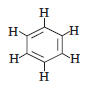
Question. Name a cyclic unsaturated carbon compound.
Answer.Benzene is cyclic unsaturated carbon compound
Question. Which of the following is not observed in a homologous series? Give reason for your choice.
(a) Change in chemical properties (b) Difference in -CH2 and 14u molecular mass
(c) Gradation in physical properties (d) Same functional group
Answer.(a) It does not occur due to the presence of the same functional group.
Short Answer Type Questions:
Question. Elements forming ionic compounds attain noble gas electronic configuration by either gaining or losing electrons from their valence shells. Explain giving reason why carbon cannot attain such a configuration in this manner to form its compounds. Name the type of bonds formed in ionic compounds and in the compounds formed by carbon. Also explain with reason why carbon compounds are generally poor conductors of electricity.
Answer: Ionic compounds are formed either by gaining or losing electrons from the outermost shells, but carbon which has four electrons in its outermost shell cannot form ionic bonds because – If carbon forms ionic bonds by gaining four electrons to attain a noble gas configuration then it would be difficult for six protons in the nucleus to hold ten electrons.
– If carbon forms ionic bonds by loss of four electrons then it would require a lot of energy to remove these electrons from outermost shell.
Due to these reasons carbon forms covalent bonds by sharing the valence electrons.
Type of bonds formed in ionic compounds are called electrovalent bonds and the type of bonds formed in carbon compounds are called covalent bonds.
Question. Write the structural formula of chloroethane.
Answer: Structural formula of chloroethane (CH3CH2Cl) is

Question. Write the names of the functional groups in :

Answer:

Question. Name the following compound :
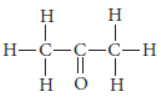
Answer: The IUPAC name of propanone.

Question. State two properties of carbon which lead to a very large number of carbon compounds.
Answer: Carbon forms a large number of carbon compounds like long chains which may be straight or branched chains or ring of different sizes due to its tetravalency and unique property of catenation. Carbon due to its small size forms exceptionally stable compounds by forming strong bonds.
Question. An aldehyde as well as a ketone can be represented by the same molecular formula, say C3H6O. Write their structures and name them. State the relation between the two in the language of science.
Answer: The aldehyde and ketone represented by the molecular formula, C3H6O.
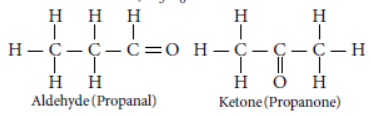
In the language of science, they are called as isomers because both have same molecular formula but different structural formulae (having different functional groups.)
Question. What is meant by isomers? Draw the structures of two isomers of butane, C4H10.
Explain why we cannot have isomers of first three members of alkane series.
Answer: Isomers are those molecules which have the same molecular formula but different structural formular i.e., show different properties.
The structures of possible isomers of butane (C4H10) are :

The first three members of alkane series are :
(i) CH4 (methane) (ii) C2H6 (ethane)
(iii) C3H8 (propane)
In the above members of alkane series, it is not possible to have different arrangements of carbon atoms. Thus, we cannot have isomers of first three members of alkane series.
Question. Write the molecular formula of the following compounds and draw their electron-dot structures :
(i) Ethane (ii) Ethene
(iii) Ethyne
Answer: (i) Molecular formula of ethane is C2H6. Its electron dot structure is :
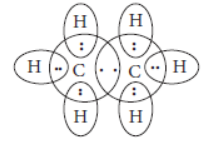
(ii) Molecular formula of ethene is C2H4.
Its electron dot structure is :

(iii) Molecular formula of ethyne is C2H2.
Its electron dot structure is :
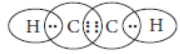
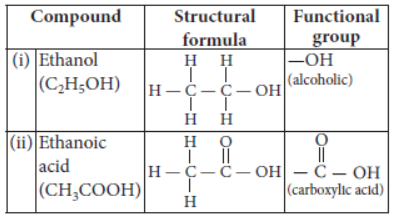
Question. What is meant by functional group in carbon compounds? Write in tabular form the structural formula and the functional group present in the following compounds :
(i) Ethanol
(ii) Ethanoic acid
Answer:
Question. State the reason why carbon can neither form C4+ cations nor C4– anions, but forms covalent compounds. Also state reasons to explain why covalent compounds :
(i) have low melting and boiling points?
Answer: (i) Covalent compounds have low melting and boiling points because the forces of attraction between molecules of covalent compounds are very weak. On applying a small amount of heat these molecular forces break.
Question. Why is homologous series of carbon compounds so called? Write the chemical formula of two consecutive members of any homologous series and state the part of these compounds that determines their (i) physical and (ii) chemical properties.
Answer: Consecutive members of the homologous series of alcohols are :
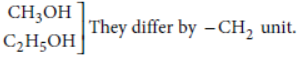
The physical properties are determined by alkyl group/hydrocarbon part/part other than the functional group.
The chemical properties are determined by functional group such as —OH group.
Question. When ethanol reacts with ethanoic acid in the presence of conc. H2SO4, a substance with fruity smell is produced. Answer the following :
(i) State the class of compounds to which the fruity smelling compounds belong.
Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of conc. H2SO4 in this reaction.
Answer: (i) When ethanol reacts with ethanoic acid in presence of conc. H2SO4 ethyl ethanoate is formed which belongs to the class of ester compounds, having fruity smell. 87
(ii) The above reaction is called esterification which occurs in presence of conc. H2SO4 which acts as a dehydrating agent and helps in the removal of water. Conc. H2SO4 also acts as a
catalyst to speed up the reaction.
Question. Write a chemical equation to represent what happens when hydrogen gas is passed through an unsaturated hydrocarbon in the presence of nickel acting as a catalyst.
Answer:

Question. State the meaning of the functional group in an organic compound. Write the formula of the functional group present in alcohols, aldehydes, ketones and carboxylic acids.
Answer: The formulae for different functional groups are :
alcohols : —OH group
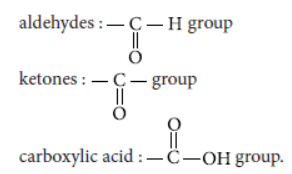
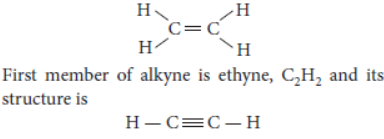
Question. What is meant by homologous series of carbon compounds? Write the general formula of (i) alkenes, and (ii) alkynes. Draw the structures of the rst member of each series to show the bonding between the two carbon atoms.
Answer: The general formula for alkenes is CnH2n and for alkynes is CnH2n – 2.
First member of alkene is ethene, C2H4 and its structure is
Question. Why does carbon form compounds mainly by covalent bonding?
Answer: As carbon has four valence electrons and it can neither loose nor gain four electrons thus, it attains noble gas configuration only by sharing of electrons. Thus, it forms covalent compounds.
Question. Why are covalent compounds generally poor conductors of electricity?
Answer: Covalent compounds are generally poor conductors of electricity because they do not have free electrons or ions.
Question. What are covalent compounds? Why are they different from ionic compounds? List their three characteristic properties.
Answer: Covalent compounds are those compounds which are formed by sharing of valence electrons between the atoms e.g., Hydrogen molecule is formed by mutual sharing of electrons between two hydrogen atoms.
They are different from ionic compounds as ionic compounds are formed by the complete transfer of electrons from one atom to another e.g., NaCl is formed when one valence electron of sodium gets completely transferred to outer shell of chlorine atom.
The characteristic properties of covalent compounds are :
(i) They are generally insoluble or less soluble in water but soluble in organic solvents.
(ii) They have low melting and boiling points.
(iii) They do not conduct electricity as they do not contain ions.
Question. What are covalent bonds? Show their formation with the help of electron dot structure of methane. Why are covalent compounds generally poor conductors of electricity?
Answer: Covalent bonds are those bonds which are formed by sharing the valence electrons between two atoms. Electron dot structure of methane is shown in the figure.

Question. Give reasons for the following :
(i) Diamond has high melting point.
(ii) Graphite is a good conductor of electricity.
Answer: (i) In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure. This makes diamond the hardest known substance. Thus, it has high melting point.
(ii) In graphite, each carbon atom is bonded to three other carbon atoms by covalent bonds in the same plane giving a hexagonal array. Thus, only three valence electrons are used for bond formation and hence, the fourth valence electron is free to move. As a result, graphite is a good conductor of electricity.
Question. Define the term ‘structural isomerism’.
Explain why propane cannot exhibit this property. Draw the structures of possible isomers of butane, C4H10.
Answer: Two or more organic compounds having the same molecular formula but different structures, are called structural isomers and the phenomenon is known as structural isomerism.
There is no possible isomers for propane as it contains three carbon atoms and it is not possible to have different arrangements of these carbon atoms.
Question. What are hydrocarbons? Distinguish alkanes from alkenes and each of them from alkynes, giving one example of each. Draw the structure of each compound cited as example to justify your answer.
Answer: Hydrocarbons are the compounds of carbon and hydrogen atoms. Those hydrocarbons which contain only single carbon-carbon bonds are called alkanes while those having double and triple bonds are called alkenes and alkynes respectively.
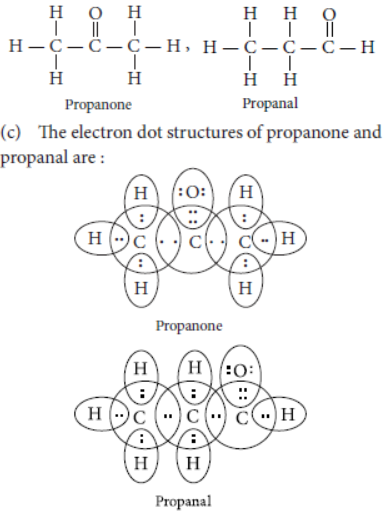
Question. (a) Draw two possible isomers of the compound with molecular formula C3H6O and write their names.
(b) Give the electron dot structures of the above two compounds.
Answer: (a) Two possible isomers of the compound, C3H6O are :
Question. Explain isomerism. State any four characteristics of isomers. Draw the structures of possible isomers of butane, C4H10.
Answer: Isomers are those compounds which have same molecular formula but different structures.
The phenomenon of existing these isomers are called isomerism.
Four characteristics of isomers are :
(i) They have same molecular formula but different structures.
(ii) Isomers is possible only with hydrocarbons having four or more carbon atoms.
(iii) Due to isomerism, a given molecular formula can represent two or more different compounds.
(iv) Due to isomerism, the different compounds have different properties.
Question. Name the process by which unsaturated fats are changed to saturated fats.
Answer: Hydrogenation is the process in which unsaturated fats are changed to saturated fats.
Question. (a) What is a homologous series of compounds? List any two of its characteristics.
(b) What is the next higher homologue of C3H7OH? What is its formula and what is it called?
Answer: Two characteristics of homologous series :
(i) The successive compounds of the homologous series differ by —CH2 unit i.e. 14 mass units.
(ii) Each homologous series belongs to similar class of compounds which shows the same properties.
(b) Next higher homologue of C3H7OH is C4H9OH i.e. butanol.
Question. What happens when 5% alkaline KMnO4 solution is added drop by drop to warm ethanol taken in a test tube? State the role of alkaline KMnO4 solution in this reaction.
Answer: When 5% alkaline KMnO4 solution is added drop by drop to warm ethanol then it gets oxidised to ethanoic acid.
CH3CH2OH alkaline → KMnO4 CH3COOH
Ethanol Ethanoic acid
Here, alkaline KMnO4 acts as an oxidising agent i.e., the substance which is capable of adding oxygen to others. Thus, alkaline KMnO4 provides oxygen to ethanol to form ethanoic acid.
Question. (a) What is a detergent? Name one detergent.
(b) Why, by using a detergent, can we wash clothes even in hard water?
Answer: (a) Detergents are ammonium or sulphonate or sulphate salts of long chain hydrocarbons containing 12-18 carbon atoms e.g., dodecyl benzene sulphonate.
(b) Synthetic detergents can be used even in hard water because they do not react with Ca2+and Mg2+ions present in hard water. They do not form curdy white precipitates (scum) of calcium and magnesium salts of fatty acids.
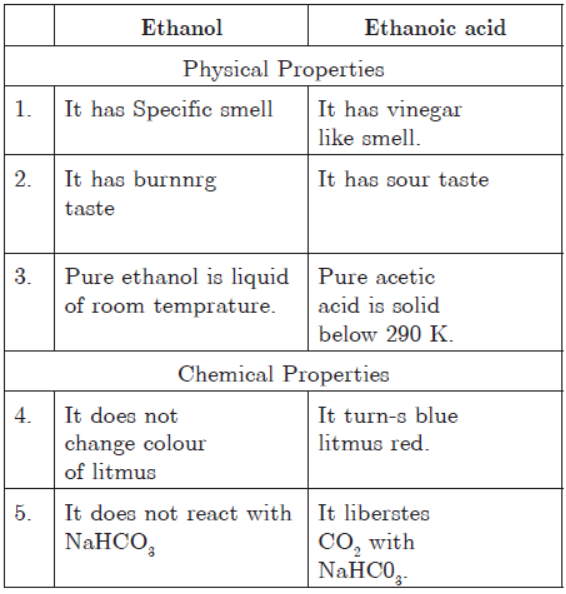
Question. List in tabular form three physical and two chemical properties on the basis of which ethanol and ethanoic acid can be differentiated.
Answer:

Question. Two carbon compounds X and Y have the molecular formula C4H8 and C5H12 respectively. Which one of these is most likely to show addition reaction? Justify your answer. Also give the chemical equation to explain the process of addition reaction in this case.
Answer: All unsaturated hydrocarbons (containing double or triple bonds) have tendency to get converted to saturated hydrocarbons (single bonds) by adding small molecules such as
hydrogen (H2), halogens (X2), etc. Such reactions are called addition reactions.
Compound X i.e. C4H8 belongs to alkene series (CnH2n) while compound Y i.e. C5H12 belongs to
alkane series (CnH2n + 2). Thus, compound X will undergo addition reaction.
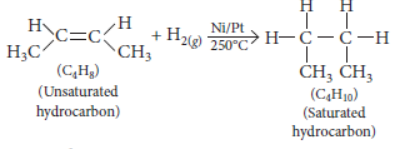
Question. The molecular formula of two carbon compounds are C4H8 and C3H8. Which one of the two is most likely to show addition reaction? Justify your answer. Also give the chemical equation to explain the process of addition reaction in this case.
Answer: C4H8 belongs to alkene series (CnH2n) while C3H8 belongs to alkane series (CnH2n + 2). Thus, C4H8 will undergo addition reaction.
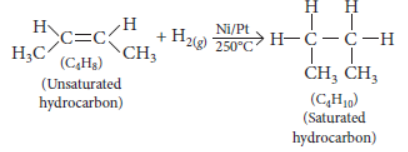
Question. What is an oxidising agent? What happens when an oxidising agent is added to propanol?
Explain with the help of a chemical equation.
Answer: The substance that supply oxygen in a reaction for oxidation is called oxidising agent e.g., potassium permanganate, potassium dichromate, etc.
When propanol is heated with alkaline KMnO4, it gets oxidised to propanoic acid.

Question. Draw the electron-dot structure for ethyne.
A mixture of ethyne and oxygen is burnt for welding. In your opinion, why cannot we use a mixture of ethyne and air for this purpose?
Answer: The formula for ethyne is C2H2 and its electron dot structure is : A mixture of ethyne and oxygen is burnt for welding so that complete oxidation of ethyne takes place. If in place of oxygen, air is taken which contains less amount of oxygen then incomplete combustion of oxygen takes place and temperature required for welding will not be attained.
Question. Write the name and general formula of a chain of hydrocarbons in which an addition reaction with hydrogen is possible. State the essential condition for an addition reaction.
Stating this condition, write a chemical equation giving the name of the reactant and the product of the reaction.
Answer: Alkene, having general formula as CnH2n and alkyne, having general formula as CnH2n – 2 are the class of hydrocarbons in which addition reaction is possible.
The essential conditions for addition reaction are :
(i) Presence of unsaturated hydrocarbon.
(ii) Presence of catalyst such as Ni/Pt/Pd.
Let us take an example of ethene. It undergoes addition reaction with hydrogen when it is heated in the presence of nickel catalyst to form ethane.
The reaction is known as hydrogenation.
CH2 CH2 + H2 Ni → Catalyst CH3 CH3
Ethene Ethane
Question. Explain why carbon forms compounds mainly by covalent bond. Explain in brief two main reasons for carbon forming a large number of compounds. Why does carbon form strong bond with most other elements?
Answer: Due to the small size of carbon atom, nucleus holds the shared pair of electrons between atoms strongly. Thus, carbon forms strong covalent bonds with elements such as hydrogen, oxygen, nitrogen, sulphur, chlorine and other elements.
Question. Name the functional groups of organic compounds that can be hydrogenated. With the help of suitable example explain the process of hydrogenation mentioning the conditions of the reaction and any one change in physical property with the formation of the product. Name any one natural source of organic compounds that are hydrogenated.
Answer: Hydrogenation is the addition of hydrogen to an unsaturated hydrocarbon to obtain a saturated hydrocarbon.

Here R can be any alkyl group.
There is the change of unsaturated compound from the liquid state to saturated compound in the solid state thus, melting point increases.
Naturally occurring oils such as groundnut oil, cotton seed oil which contain double bonds can be easily hydrogenated.
Question. What are hydrocarbons? Write the name and general formula of
(i) saturated hydrocarbons
(ii) unsaturated hydrocarbons, and draw the structure of one hydrocarbon of each type. How can an unsaturated hydrocarbon be made saturated?
Answer: Organic compounds containing carbon and hydrogen are called hydrocarbons.
(i) Name and general formula of saturated hydrocarbons is

Question. (a) How does a saturated hydrocarbon react with chlorine? Write chemical equation for it. What type of reaction is it called and why?
Answer: (a) Saturated hydrocarbon reacts with chlorine to form a substituted product. e.g.,
CH4 + Cl2 hv → CH3Cl+HCl
Methane
This reaction is called substitution reaction as here one hydrogen of methane is substituted by one chlorine atom.
Question. Draw the structure for ethanoic acid molecule, CH3COOH.
Answer: Structure of ethanoic acid is

Question. What happens when a small piece of sodium is dropped into ethanol?
Answer: When a small piece of sodium is dropped into ethanol then hydrogen gas is liberated which burns with a pop sound.
2C2H5OH + 2Na → 2C2H5O–Na+ + H2↑
Question. Name the gas evolved when ethanoic acid is added to sodium carbonate. How would you prove the presence of this gas?
Answer: When ethanoic acid is added to sodium carbonate, CO2 gas is evolved which turns lime water milky.
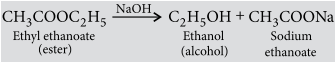
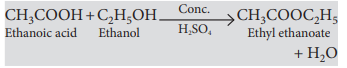
Question. Write the chemical equations to show what happens when
(i) an ester reacts with a base?
(ii) ethanol reacts with ethanoic acid in the presence of sulphuric acid?
Answer: (i) When an ester reacts with the base then it gives sodium salt of carboxylic acid and an alcohol. It is known as saponification reaction.
(ii) Carboxylic acids react with alcohols in the presence of a little concentrated sulphuric acid to form pleasant smelling esters. This reaction is called esterification reaction.
Question. Write the respective chemical equations to show what happens when
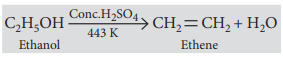
(i) ethanol is heated with concentrated sulphuric acid at 443 K ?
Answer: (i)
Question. Give reasons for the following observation :
Air holes of a gas burner have to be adjusted when the heated vessels get blackened by the flame.
Answer: Air holes of a gas burner have to be adjusted when the heated vessels get blackened by the flame as to get sufficient supply of oxygen (air) for complete combustion.
Question. Complete the following chemical equations:
(i) C2H5OH + O2 →
(ii) C2H5OH Conc.H2SO4 → 443k
(iii) CH3COOH+NaHCO3 →
Answer:

Question. Write the structural formula of ethanol.
What happens when it is heated with excess of conc. H2SO4 at 443 K? Write the chemical equation for the reaction stating the role of conc. H2SO4 in this reaction.
Answer: The structural formula of ethanol (C2H5OH) is
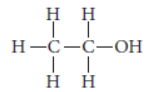
When ethanol is heated with conc. H2SO4 at 443 K then it loose a water molecule to form unsaturated alkene (ethene) as a product.
CH3CH2OH conc. H2SO4 → 443 K CH2 = CH2 + H2O
Here conc. H2SO4 acts as a dehydrating agent i.e., helps in the removal of water.
Question. Distinguish between esterification and saponification reaction with the help of the chemical equations for each. State one use of each (i) esters, and (ii) saponification process.
Answer:
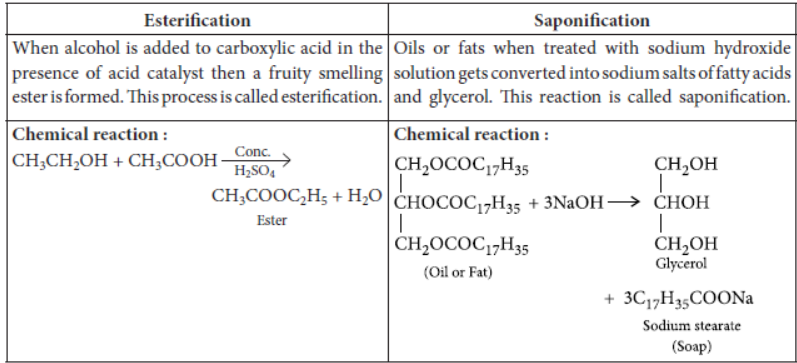
Use of esters : They are used for making perfumes or used as artificial faavouring substances.
Use of saponification process : This process is used in making soaps.
Question. Explain esterification reaction with the help of a chemical equation. Describe an activity to show esterification.
Answer: Aim : To demonstrate esterification process using ethanol and acetic acid.
Materials required : Beaker, water, test tube, ethanol, acetic acid, conc. H2SO4, tripod stand, burner, wire gauze, etc.
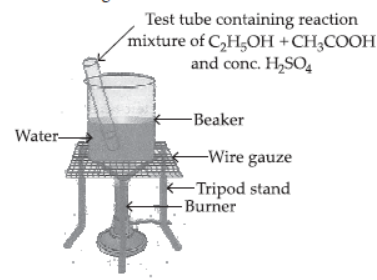
Procedure :
– Take 2 mL of ethanol in a test tube.
– Take 2 mL of ethanoic acid (acetic acid) into it.
– Add few drops of conc. H2SO4.
– Warm it in a beaker containing water.
– Observe the smell of the products formed.
Observations : Pleasant fruity smelling compound
(called ester) is formed.
Chemical reaction :
CH3COOH(l) + C2H5OH(l) Conc. H2SO4 → CH3COOC2H5 + H2O
Ethanoic acid Ethanol Ethyl ethanoate Water
Conclusion : Carboxylic acid reacts with alcohol in presence of conc. H2SO4 which acts as a dehydrating agent to form esters.
Question. Give reason for the following observation :
Use of synthetic detergents causes pollution of water.
Answer: Synthetic detergents causes water pollution because they cannot be easily decomposed by microorganisms like bacteria.
Question. Explain the cleansing action of soap.
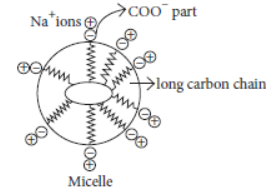
Answer: Cleansing action of soap : When a dirty cloth is put in water containing dissolved soap or detergent then the new polar hydrocarbon tails of the soap or the detergent dissolve oil or grease while the ionic parts of soap or detergent (having negative charge) get attach to the water molecules. As a result, soap or detergent micelles are formed in which the soap or detergent molecules are arranged radially with hydrocarbon ends directed towards the centre and ionic ends directed outwards. In this way, micelle entraps the oily or greasy dirt by using its hydrocarbon ends.
The ionic ends remain attached to water. When the surface of the cloth is scrubbed or agitated the loosened oily dirt particles are removed and cloth is cleaned.

Question. Why does micelle formation take place when soap is added to water? Why are micelles not formed when soap is added to ethanol?
Answer: A soap molecule has two ends with different properties, one end is polar i.e., water soluble or hydrophilic while other end is non-polar i.e., water insoluble or hydrophobic. When soap is added to water, the polar ends get dissolve in water and non-polar ends get dissolve in each other and directed towards the centre. As a result, a spherical ionic molecule known as micelles, formation takes place. Since, soaps are soluble in ethanol, therefore, micelles formation does not occur.
Question. Soaps and detergents are both, types of salts.
State the difference between the two. Write the mechanism of the cleansing action of soaps. Why do soaps not form lather (foam) with hard water? Mention any two problems that arise due to the use of detergents instead of soaps.
Answer: Soaps are the sodium or potassium salts of higher fatty acids. The ionic group in soaps is –COO–Na+.
On the other hand, synthetic detergents are the sodium salts of a long chain alkylbenzenesulphonic acids or long chain alkyl hydrogen sulphates. The ionic group in synthetic detergents is –SO3 – Na+ or –OSO3 –Na+
Cleansing action of soap :
A soap molecule contains a polar part (COO–Na+) called polar end and a non-polar part consisting of a long chain carbon atoms. This part is called hydrocarbon end.
The polar end is water soluble whereas hydrocarbon part is water-repellent and oil soluble.
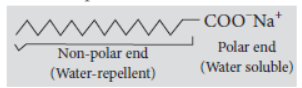
When an oily (dirty) piece of cloth is put into soap solution, the hydrocarbon part of the molecule attaches itself to the only drop and the –COO– end orients itself towards water. Na+ ions in solution arrange themselves around the –COO– ions. The negatively charged micelle so formed entraps the oily dirt.
The negatively charged micelle repel each other due to the electrostatic repulsion. As a result, the tiny oily dirt particles do not come together and get washed away in water during rinsing.
In hard water, soap does not form lather as hard water contains Ca2+and Mg2+ ions. Soap reacts with these ions to form insoluble calcium and magnesium salts of fatty acids.
RCOO–Na+ + Ca2+ (aq) → (RCOO)2Ca↓ + 2Na+
Soap Insoluble ppt.
Two problems which arise due to the use of detergents instead of soaps are :
(i) Synthetic detergents are non-biodegradable and hence, cause water pollution.
(ii) Synthetic detergents also cause skin related problems.
Question. (a) You have three unlabelled test tubes containing ethanol, ethanoic acid and soap solution. Explain the method you would use to identify the compounds in different test tubes by chemical tests using litmus paper and sodium metal.
(b) Give the reason of formation of scum when soaps are used with hard water.
Answer: (a) The tests may be tabulated as below :

(b) Hard water contains hydrogen carbonates, chlorides and sulphates of calcium and magnesium.
When soap is added to hard water it reacts with these salts to form scum which is insoluble in water and foats on the top of the water surface. The scum is formed due to the formation of insoluble calcium or magnesium salts of fatty acids.
2C17H35COONa + Ca2+ → (C17H35COO)2Ca
Sodium stearate (From Calcium stearate
(soap) hard water) (ppt. or scum) + 2Na+
Question. A carbon compound ‘P’ on heating with excess conc. H2SO4 forms another carbon compound ‘Q’ which on addition of hydrogen in the presence of nickel catalyst forms a saturated carbon compound ‘R’. One molecule of ‘R’ on combustion forms two molecules of carbon dioxide and three molecules of water. Identify P, Q and R and write chemical equations for the reactions involved.
Answer: When ethanol is heated with excess of concentrated H2SO4 it gets dehydrated to form ethene.

When ethene is heated with hydrogen in presence of nickel catalyst it forms ethane.
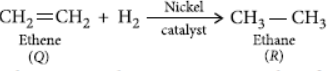
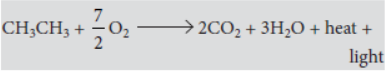
Ethane on oxidation gives two moles of carbon dioxide and three moles of water.
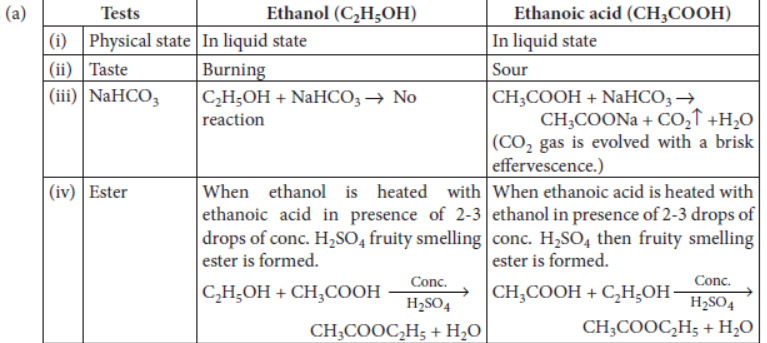
Question. (a) In a tabular form, differentiate between ethanol and ethanoic acid under the following heads :
(i) Physical state (ii) Taste
(iii) NaHCO3 test (iv) Ester test
(b) Write a chemical reaction to show the dehydration of ethanol.
Answer:
Question. What are detergents chemically? List two merits and two demerits of using detergents for cleansing. State the reason for the suitability of detergents for washing, even in the case of water having calcium and magnesium ions.
Answer: Detergents are generally ammonium or sulphonate salts of long chain carboxylic acids.
They are sodium salts of long chain alkyl benzene sulphonic acids.
Merits of using detergents :
(i) Detergents are very strong cleansing agents.
(ii) They can form lather well even in hard water as they do not form insoluble calcium or magnesium salts.
Demerits of using detergents :
(i) As detergents are sodium salts of long chain alkyl benzene sulphonic acids which are very bulky molecules, are not easily degraded by bacteria and hence, they are non-biodegradable.
(ii) They are highly basic in nature and cause damage to skin.
Question. The general formula of three compounds A, B and C is CnH2n. ‘B’ has the highest boiling point and ‘C’ has the lowest boiling point.
(i) Mention the type of compounds A, B and C.
(ii) Which of these has minimum number of carbon atoms?
(iii) Name the homologous series to which A, B and C belong
Answer.
(i) Unsaturated hydrocarbons with double bonds.
(ii) ‘C’ has minimum boiling point, so ‘C’ has minimum no. of C-atoms.
(iii) Alkene
Question. Select alkenes and alkynes from the following:
C2H4, C3H4, C2H2, C4H8
Write their structural formula also.
Answer. C2H4 and C4H8 are alkenes, C3H4 and C2H2 are alkynes.
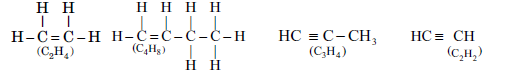
Question. An alkane has molecular weight 86. Write its molecular formula. What will be its physical state?
Answer.C6H14 has molecular weight of 6 × 12 + 14 = 86u.
It is in liquid state at room temperature.
Question.1. What is homologous series of carbon compounds? Give an example and list its three characteristics.
Answer.
1. The series of organic compounds having same functional group and similar chemical properties is called homologous series. For example,

Characteristics:
• Each successive member differ by CH2 unit.
• They have gradation in physical properties.
• They have similar chemical properties due to presence of same functional group.
Question: Unsaturated hydrocarbons contain multiple bonds between the two c-atoms and show addition reaction. Give the test to distinguish ethane from ethene.
Answer: To distinguish between saturated and unsaturated hydrocarbons, a combustion test should be performed.
A saturated hydrocarbon undergoes complete combustion and burns with blue flame.
Also, it does not leave any residue behind after burning. On the contrary, an unsaturated hydrocarbon undergoes incomplete combustion and burns with yellow flame. Also, it leaves some residue behind after burning. Ethane is a saturated hydrocarbon. Thus, it burns with a blue flame and does not leave a residue behind.
Ethene is an unsaturated hydrocarbon. Thus, it burns with a yellow flame and leaves some residue behind.
Question. a. Write chemical name and formula of vinegar?
b. Describe with a chemical equation what happens when sodium reacts with ethanol.
Answer:
a. Vinegar contains ethanoic acid,
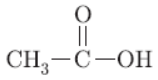
b. Sodium ethoxide and hydrogen gas is formed.
2C2H5OH + 2Na → 2C2H5ONa + H2
Question. Explain giving reasons, why carbon can neither form C4+ cation nor C4- anion but forms covalent compounds which are bad conductors of electricity and have low melting and boiling points.
Answer: Carbon cannot lose four electrons because high energy is needed to remove four electrons. It cannot gain 4 electrons because 6 protons cannot hold 10 electrons.
It can share 4 electrons to form covalent bonds.
Covalent compounds do not conduct electricity because these do not form ions. They have low melting and boiling points due to weak force of attraction between molecules.
Question. List two characteristics of covalent compounds.
Answer:
(i) They have low melting and boiling point.
(ii) They do not conduct electricity.
Question. The structural formula of an ester is
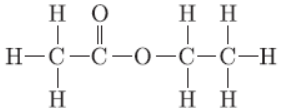
Write the structural formula of the corresponding alcohol and acid.
Answer:

Question. Name an element, other than carbon, which exhibits property of catenation up to seven or eight atoms. Are these compounds stable?
Answer: Si and Sulphur (S8).
No, these compounds are not stable, rather they are reactive.
Question. Select alkenes and alkynes from the following:
C2H4, C3H4, C2H2, C4H8
Answer: Alkenes C2H4, C4H3 Alkynes C3H4, C2H2
Question. Why are detergents preferred over soaps for washing clothes in hard water? Explain.
Answer: Detergents work well even with hard water because their calcium and magnesium salts are soluble in water. They do not form scum.
Question. What happens when ethyl alcohol and acetic acid react with each other in presence of cone. H2SO4?
Answer: Pleasant fruity smelling compound ester is formed cone. H2SO4.
CH3COOH + C2H5OH → Cone.H2SO4
Δ
CH3COOC2H5 + H2O
Question. Name the functional groups of the following compounds:

Answer:
a. Carboxylic acid
b. Ester
c. Alcohol
d. Halogen
Question. Why is hydrogenation? What is its industrial application?
Answer: Hydrogenation is a process of adding hydrogen to unsaturated compounds in presence of catalyst like nickel to form saturated hydrocarbons. Industrially, it is used to convert vegetable oils to vegetable ghee.
Question. List four characteristics of homologous series.
Answer:
a. All members are derived from same general formula.
b. All members have same functional group.
c. Each successive member differ by —CH2 unit.
d. All members can be prepared by same methods of preparation.
Question. Carbon does not form ionic compounds, why?
Answer: Carbon cannot lose four electrons because high energy is needed to remove four electrons. It cannot gain 4 electrons because 6 protons cannot hold 10 electrons.
That is why carbon cannot form ionic compounds.
Question. Draw the structural formulae of the possible isomers for the compound with molecular formula C3H6O.
Answer:
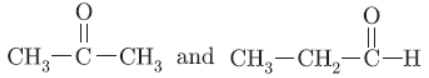
Question. Name two oxidising agents that are used to convert alcohols to acids. Distinguish between ethanol and ethanoic acid on the basis of (a) litmus test (b) reaction with NaHCO3.
Answer: These two oxidising agents will convert alcohols to acids (i) Alkaline KMnO4 (ii) Acidified K2Cr2O7
a. Litmus test: Acetic acid turns blue litmus red but ethanol does not.
b. NaHCO3 test: Acetic acid will give brisk effervescence due to evolution of CO2 whereas ethanol will not react.
Question. Explain why cannot we have isomers of first three members of alkane family.
Answer: It is because branching is not possible with carbon atoms, that is why, there are no isomers till propane.
Question. Write balanced equations for the burning of (a) methane (b) ethane in air.
Answer:
(a) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
(b) 2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(l)
Question. How do the melting and boiling points of the hydrocarbons change with increase in molecular mass?
Answer: Melting and boiling point of the hydrocarbons increases with increase in molecular mass because surface area increases which results an increase in vander Waal’s forces of attraction between molecules.
Question. Write a chemical test to distinguish between ethanol and ethanoic acid.
or
How would you distinguish experimentally between ethanol and ethanoic acid with the help of sodium hydrogen carbonate? Write the chemical equation for the reaction involved.
Answer: Add NaHCO3 to each of them separately. Ethanol will not react. Ethanoic acid will give brisk effervescence due to CO2.
CH3COOH + NaHCO3 →
CH3COONa + H2O + CO2
C2H5OH + NaHCO3 → No reaction
Question. Explain the action of soap in removing an oily spot from a piece of cloth.
Answer: Cleansing action of soap: Soap has ionic end which is hydrophilic, interacts with water while carbon chain is hydrophobic interacts with oil, grease. The soap molecules orient themselves in a cluster in which hydrophobic tails are inside the cluster and ionic ends face outside.
These cluster are called micelles. These attract oil which is washed away by water.
Question. Draw electron dot structures of (i) C2H4 (ii) C2H5OH.
Answer:
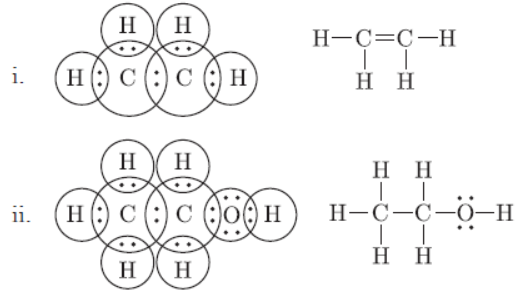
Question. Write the molecular formula of benzene and draw its structure. List in tabular form how covalent compounds differ from ionic compounds.
Answer:

Question. State reasons to explain why covalent compounds:
a. are bad conductors of electricity?
b. have low melting and boiling points?
Answer:
a. Covalent compounds do not form ions, hence they are bad conductor of electricity.
b. Covalent compounds have weak intermolecular forces of attraction, therefore, have low melting and boiling points.
Question. What are soaps? Why do they form scum with hard water?
Answer: Soaps are sodium or potassium salts of fatty acids e.g. sodium stearate. They react with Ca2+ and Mg2+ ions in hard water to form calcium and magnesium salt of fatty acids which are insoluble in water and called scum.
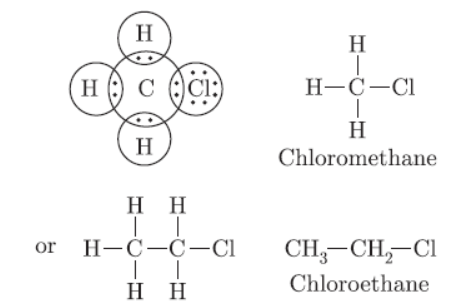
Question. Give the electron dot structure of chloro-methane. Also write the formula and the name of next homologue of it.
Answer:
Question. Distinguish between esterification and saponification reaction with the help of equations for each. State one use of each (i) esters and (ii) saponification process.
Answer: Esterification
C2H5OH + CH3COOH Cone.H2SO4→ CH3COOC2H5 + H2O
Ester
Saponification
CH3COOC2H5 + NaOH →
CH3COONa + C2H5OH
Esters are used in synthetic flavours, perfumes, etc.
Saponification process is used for manufacture of soaps.
Long Answer Type Questions:
Question: A reaction is commonly used in the conver- sion of vegetable oils to fats. Explain the re- action involved in detail.
Answer: The reaction that is used to convert vegetable oil to fat is called as hydrogenation reaction.
Vegetable oils contain unsaturated hydrocarbons which can exhibit addition reaction with hydrogen to form saturated hydrocarbons or fats. The reaction occurs in the presence of metal catalyst like nickel or palladium at 200 o C and forms saturated vegetable fats.
The hydrogenation reaction is an industrial method for manufacturing vanaspati ghee from vegetable oil.

Question: What is a homologous series of carbon compounds? List its any two characteristics.
Write the name and formula of the next higher homologous of HCOOH.
Answer: Homologous series is a series of carbon compounds in which the hydrogen in a carbon chain is replaced by the same functional group.
Characteristics of homologous series are:
(1) All members of a homologous series can be represented by the same general formula.
For ex, the general formula for alkanes is CnH2n+2, where n is the number of carbon atoms.
(2) They have similar chemical properties.
(3) Any two ad??acent homologues differ by CH2 in their molecular formula.
(4) The difference in the molecular masses of any two adjacent homologues is 14 u.
(5) All the compounds belonging to the same homologous series have similar chemical properties.
(6) The members of a homologous series show a gradual change in their physical properties with increase in molecular mass.
The name and molecular formula of the next two members of the homologous series having compound HCOOH is:
(i) Ethanoic acid, CH3COOH
(ii) Propanoic acid, C2H5COOH
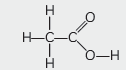
Question: The formulae of four organic compounds are given below:

(A) Which one of these compounds A, B, C or D is a saturated hydrocarbon?
(B) Identify the organic acid and give its structural formula.
Answer: (A) D is a saturated hydrocarbon
(B) B is an organic acid. Structural formula:
Question. Why are certain compounds called hydrocarbons?
Write the general formula for homologous series of alkanes, alkenes and alkynes and also draw the structure of the first member of each series. Write the name of the reaction which converts alkene into alkane. Also write the chemical equation to show the necessary conditions for the reaction to occur.
Answer: Compounds of carbon and hydrogen are called hydrocarbons.
Alkane CnH2n+2
Alkyne CnH2n–2 HC ≡ CH Ethyne
Hydrogenation i.e. addition of H2 leads to formation of alkanes from alkenes.
CH2 = CH2 + H2 Ni → heat CH3 – CH3
Question. Complete the following chemical equations and write the chemical name of the products formed.
a. CH2=CH2 + H2 →
b. CH3COOH + NaOH →
c. CH3CH2OH H2SO4
d. HCOOH + Na →
e. C2H5OH Alk MnO4 →
Answer:
a. CH2= CH2 + H2 → CH3– CH3
b. CH3COOH + NaOH →
CH3COONa + H2O
Sodium ethanoate
c. CH3CH2OH Conc.H2SO4 → Δ CH2= CH2 + H2O
Ethene
d. 2HCOOH + 2Na → 2HCOONa + H2
Sodium methanoate
e. C2H5OH Alk.KMnO4 → CH3COOH + H2O
Ethanoic acid
Question. a. Give a chemical test to distinguish between saturated and unsaturated hydrocarbons.
b. Name the products formed when ethane burns in air. Write the balanced chemical equation for the reactions showing two types of energies liberated.
c. Why is reaction between methane and chlorine in presence of sunlight is considered a substitution reaction.
Answer:
a. Saturated hydrocarbons will not react with bromine water whereas unsaturated hydrocarbons will decolourise it.
b. Carbon dioxide and water will be formed.
2C2H6 + 7O2 → 4CO2(g) + 6H2O(l)
+ Heat + Light
c. It is because hydrogen atom is substituted by halogen atom, that is why it is called substitution reaction.
Question. a. You have three unlabelled test tubes containing ethanol, ethanoic acid and soap solution. Explain the method you would use to identify the compounds in different test tubes by chemical tests using litmus paper and sodium metal.
b. Give reason of formation of scum when soaps are used with hard water.
Answer:
a. Red litmus paper will become blue in soap solution only. Ethanoic acid will turn blue litmus red only.
Ethanol will react with Na metal to form sodium ethoxide and hydrogen gas will be liberated.
b. Soaps are sodium or potassium salts of fatty acids which react with Ca2+ and Mg2+ ions in hard water to form calcium or magnesium salts of fatty acids which are insoluble in water called scum.
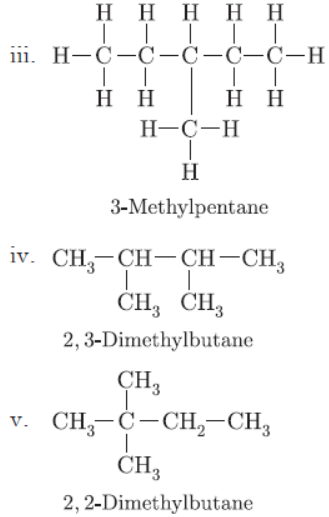
Question. You are given balls and stick model of six carbon atoms and fourteen hydrogen atoms and sufficient number of sticks. In how many ways one can join the models of six carbon atoms and fourteen hydrogen atoms to form different molecules of C6H14.
Answer: There are five ways in which six carbons can be joined with 14 hydrogen atoms.

Question. What are micelles? Why does it form when soap is added to water? Will a micelle be formed in other solvents such as ethanol also? State briefly how the formation of micelles help to clean the clothes having oily spots.
Answer:
Micelles are cluster of molecules in which hydrophobic tails are inside the cluster 3 and the ionic ends are at the surface of clusters. Soap molecules when dissolved in water they form a cluster due to hydrophobic part of molecules orient themselves away from water.
So they arrange towards inside of the cluster while hydrophilic part remain outside of cluster.
No, micelles will not be formed in alcohol. Soap in form of micelles is able to clean because the oily dirt will be collected in centre of micelle which is rinsed away by water.
Question. a. Differentiate between soap and detergent.
b. Explain why, soaps form scum with water whereas detergent do not.
Answer:
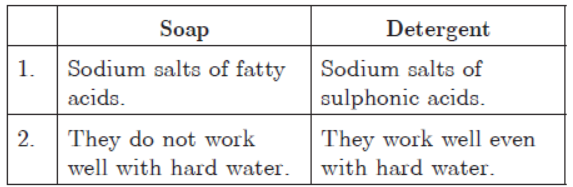
b. Sodium salts of fatty acids (soaps) react with Ca2+ and Mg2+ ions in hard water to form insoluble salts called scum. Detergents form soluble salts with Ca2+ and Mg2+.
Question. What are detergents chemically? List two merits and two demerits of using detergents for cleansing. State the reason for the suitability of detergents for washing even in case of water having calcium and magnesium ions.
Answer:
Detergents are sodium or potassium salts of sulphonic acids of benzene or sulphates of unsaturated hydrocarbons like alkenes with —SO3Na or —SO4Na group.
Merits:
They are more effective than soaps.
They work well even with hard water.
Demerits:
a. They are expensive.
b. Some of them create water pollution.
Question. a. How is vinegar made?
b. What is glacial acetic acid? What is its melting point?
c. Why are carboxylic acids called weak acids?
d. Write the name and formula of compounds formed when the ester CH3COOC2H5 undergoes saponification.
Answer:
a. Vinegar is 5-8% solution of acetic acid (Ethanoic acid) in water. It can be made by fermentation of ethanol in presence of oxygen.
b. Glacial acetic is pure (100%) acetic acid. Its melting point is 290 K.
c. They do not ionise completely in aqueous solution.
d. CH3COOC2H5 + NaOH →
CH3COONa + C2H5OH
Sodium ethanoate Ethanol
Question. a. How will you bring out following reactions? Write the concerned chemical reaction.
(1) Ethanol to ethene
(2) Ethanol to ethanoic acid
b. Give one example with chemical equation for the following reactions:
(1) Substitution reaction
(2) Saponification reaction
(3) Combustion reaction
Answer:
a. (1) CH3CH2OH Conc.H2SO4 → Δ CH2= CH2 + H2O
Ethanol Ethene
(2) CH3CH2OH K2Cr2O7/Conc.H2SO4 →
CH3COOH + H2O
b. (1) Substitution reaction
CH4 + Cl2 Sunlight → CH3Cl + HCl
(2) Saponification reaction
CH3COOCH3 + NaOH →
CH3COONa + CH3OH
(3) Combustion reaction
CH4 + 2O2 → CO2 + 2H2O
Question. Soaps and detergents are both types of salts. State the difference between the two. Write the mechanism of the cleansing action of soaps. Why do soaps not form lather (foam) with hard water? Mention any two problems that arise due to the use of detergents instead of soaps.
Answer:
a. Soaps are sodium or potassium salts of fatty acids e.g. —COONa. Detergents are sodium or potassium salts of sulphonic acids e.g. —SO3Na or —SO4Na
b. Soaps are sodium or potassium salts of fatty acids. They contain —COONa group. Detergents are sodium or potassium salts of sulphonic acids.
They contains —SO3Na or —SO4Na group. Soap has ionic end which is hydrophilic, interacts with water while carbon chain is hydrophobic interacts with oil, grease. The soap molecules orient themselves in a cluster in which hydrophobic tails are inside the cluster and ionic ends face outside.
These cluster are called micelles. These attract oil which is washed away by water.
c. Soaps react with Ca2+ and Mg2+ ions in hard water to form calcium or magnesium salts of fatty acids which are insoluble in water and thus interfere in action of soap,
d. (i) Detergents are more expensive than soaps.
(ii) Some detergents are not biodegradable i.e. will create pollution.
Question. Identify the compounds ‘A’ to ‘E’ in the following sequence:
a. CH3CH2OH KMNO4 KOH → dil HCl A + H2O
b. CH3CH2OH + A Conc.H2SO4 → Δ B + H2O
c. B + NaOH → C + CH3CH2OH
d. A + NaHCO3 → C + D + H2O
e. CH3CH2OH + E → CH3CH2ONa + H2
Answer:
a. CH3CH2OH KMNO4 KOH → dil HCl CH3COOH + H2O
‘A’
b. CH3CH2OH + CH3COOH Conc.H2SO4 → Δ
H3COOCH2CH3 + H2O
c. CH3COOCH2CH3 + NaOH →
CH3COONa + CO2 + H2O
e. 2CH3CH2OH + 2Na → 2CH3CH2ONa + H2 ‘E’ A is CH3COOH, ‘B’ is CH3COOCH2CH3, *C’ is CH3COONa, ‘D’ is CO2, ‘E’ is Na (Sodium metal).
Question. a. In a tabular form, differentiate between ethanol and ethanoic acid under the following heads:
(i) Physical state
(ii) Taste
(iii) NaHCO3 test
(iv) Ester test
b. Write a chemical reaction to show dehydration of ethanol.
Answer:
(i)

(ii) CH3CH2OH + Conc.H2SO4 → 443K CH2=CH2 + H2O
Question. Give reasons for the following:
a. Element carbon forms compound mainly by covalent bonding.
b. Diamond has high melting point.
c. Graphite is good conductor of electricity.
d. Acetylene bums with sooty flame.
e. Kerosene does not decolourise bromine water whereas cooking oil does.
Answer:
a. It is because carbon can neither lose 4 electrons nor gain 4 electrons. It can share four electrons to form covalent bonds.
b. Diamond has strong C—C bonds and compact 3-D structure in which one carbon atom is covalently bonded to other four carbon atoms therefore, has high melting point.
c. In graphite, one carbon atom is bonded to other three carbon atoms. Remaining one electron on each carbon is free to move due to which graphite conducts electricity.
d. Acetylene has high carbon content, therefore, partial oxidation causes it to bum with sooty or smoky flame.
e. Kerosene is a saturated compound, therefore, does not decolourise bromine water.
Question. An organic compound “X’ on heating with cone. H2SO4 forms a compound ‘Y’ which on addition of one molecule of hydrogen in the presence of nickel forms a compound ‘Z’. One molecule of compound ‘Z’ on combustion forms two molecules of CO2 and three molecules of H2O. Identify giving reasons the compounds X’, ‘Y’ and ‘Z’. Write the chemical equations for all the chemical reactions involved.
Answer:

C2H6 + (7/2)O2 → 2CO2 + 3H2O
‘Z’ on combustion gives 2CO2 and 3H2O so hydrocarbon ‘Z’ must be ethane. “Y’ on addition of H2 gives ethane so ‘Y’ must be ethene. “X’ in presence of cone. H2SO4 dehydrates to ethene i.e., ‘X’ is ethanol.
Question. List in tabular form three physical and two chemical properties on the basis of which ethanol and ethanoic acid can be differentiated.
Answer:
Question: What are structural isomers? Why are isomers of first three members of alkane series not possible?
Write the all possible structures of isomer of fourth member of this series.
Answer: Structural Isomers: Compounds having same structural formula but different molecular structures are known as structural isomers.
Isomerism is not possible in first 3 members of alkane because branching is not possible, all the carbon atoms are linked in one chain only and there is not any possibility of formation of any side chain.
Therefore, no branching is possible for these three elements. Isomers of butane are: There are 2 isomers are possible.


