Sample Paper Class 12 Chemistry Term 1 Set B
Please refer to Sample Paper Class 12 Chemistry Term 1 Set B with solutions provided below. We have provided CBSE Sample Papers for Class 12 Chemistry as per the latest paper pattern and examination guidelines for Standard 12 Chemistry issued by CBSE for the current academic year. The below provided Sample Guess paper will help you to practice and understand what type of questions can be expected in the Class 12 Chemistry exam.
CBSE Sample Paper Class 12 Chemistry for Term 1 Set B
Section ‘A’
1. Phosphorus (P2) is converted into P4 while nitrogen forms N2, because :
(A) pπ – pπ-bonding is absent.
(B) pπ – pπ-bonding is weak.
(C) Phosphorus shows triple bonding.
(D) Multiple bond is formed easily.
Answer
B
2. Solid A is very hard electrical insulator in solid as well as in molten state and melts at an extremely high temperature. What type of solid is it ?
(A) Ionic solid
(B) Molecular solid
(C) Covalent solid
(D) Metallic solid
Answer
C
3. Which of the following units is useful in relating concentration of solution with its vapour pressure ?
(A) Mole fraction
(B) Parts per million
(C) Mass percentage
(D) Molality
Answer
A
4. The correct order of the packing efficiency in different types of unit cells is ________.
(A) fcc < bcc < simple cubic
(B) fcc > bcc > simple cubic
(C) fcc < bcc > simple cubic
(D) bcc < fcc = simple cubic
Answer
B
5. Which of the following is halogen exchange reaction ?
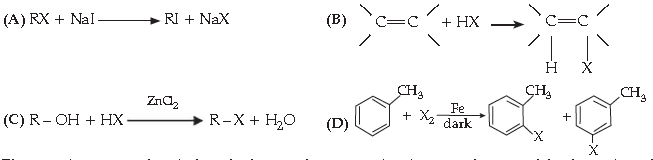
Answer
A
6. The most important chemical method to resolve a racemic mixture makes use of the formation of :
(A) a meso compound
(B) enantiomers
(C) diastereomers
(D) racemetes
Answer
C
7. Arrange the following compounds in the increasing order of their densities :

(A) (i) < (ii) < (iii) < (iv)
(B) (i) < (iii) < (iv) < (ii)
(C) (iv) < (iii) < (ii) < (i)
(D) (ii) < (iv) < (iii) < (i)
Answer
A
8. Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is :
(A) Electrophilic elimination reaction
(B) Electrophilic substitution reaction
(C) Free radical addition reaction
(D) Nucleophilic substitution reaction
Answer
C
9. When alcohol is added with petrol and is used as fuel, the two products formed are :
(A) only oxygen
(B) only water
(C) only carbon -dioxide
(D) both carbon dioxide and water
Answer
D
10. Which stoichiometric defect does not change the density of the crystal ?
(A) Frenkel defect
(B) Schottky defect
(C) Interstitial defect
(D) F-centres
Answer
A
11. In monohaloarenes, halogen atoms increases the density at which position, that leads to electrophilic substitution :
(A) ortho only
(B) para only
(C) ortho and para
(D) ortho and meta
Answer
C
12. When each sphere is in contact with four other spheres two on sides, one above and one below. The coordination number becomes four.
The close packing explained above is of which type :
(A) AAA type packing
(B) hexagonal close packing
(C) square close packing
(D) tetrahedral packing
Answer
A
13. Conversion of alcohol to alkyl halides involves :
(A) substitution reaction
(B) dehydrohalogenation reaction
(C) rearrangement reaction
(D) addition reaction
Answer
A
14. Chloropicrin is formed by the reaction of :
(A) Nitric acid on chloroform
(B) Nitric acid on picric acid
(C) Chlorine on picric acid
(D) Picric acid on chlorobenzene
Answer
A
15. Hydrolysis of starch provides us :
(A) only glucose
(B) only fructose
(C) Both glucose and fructose
(D) Glucose , fructose and sucrose
Answer
A
16. Which of the following will form alkenes most readily by acid catalysed dehydration ?
(A) (CH3)2CHCH2OH
(B) CH3CH2CH2OH
(C) CH3CHOHCH4
(D) (CH3)3COH
Answer
D
17. Which of the following correctly explains dehydration of alcohols :
(A) Addition
(B) Rearrangement
(C) Elimination
(D) Substitution
Answer
C
18. In which of the following pairs, the two species are isostructural :
(A) BrO3– and XeO3
(B) BF3 and NF3
(C) SF4 and XeF4
(D) SO32– and NO3–
Answer
A
19. In which mode of expression, the concentration of a solution remains independent of temperature ?
(A) Molarity
(B) Normality
(C) Formality
(D) Molality
Answer
D
20. Which of the following element gives two gaseous products when oxidized by conc.H2SO4 :
(A) Zn
(B) Cu
(C) C
(D) S
Answer
C
21. Function of enzymes present in the living system is to :
(A) provide energy
(B) catalyse biological reaction
(C) provide immunity
(D) transport oxygen
Answer
C
22. Out of the following statements which of the following is incorrect for nitrogen ?
(A) Its electronegativity is very high
(B) It is a typical non-metal
(C) Its molecular size is small
(D) d-orbitals are available for bonding
Answer
D

(A) C6H5OC2H5
(B) C2H5OC2H5
(C) C6H5OC6H5
(D) C6H5I
Answer
A
24. Which of the following statements is incorrect for ozone ?
(i) It oxidizes mercury
(ii) Ozone layer does not permit infra red radiation from the sun to reach the Earth.
(iii) It cannot act as bleaching agent in dry state .
(A) (i)and (ii)
(B) (ii) and (iii)
(C) only (i)
(D) only (iii)
Answer
B
25. Which of the following aqueous solutions should have the highest boiling point ?
(A) 1.0 M NaOH
(B) 1.0 M Na2SO4
(C) 1.0 M NH4NO3
(D) 1.0 M KNO3
Answer
B
Section ‘B’
26. Which of the following alcohol gives carboxylic acid with same number of carbon atom on oxidation:
(A) Primary alcohol
(B) Secondary alcohol
(C) Tertiary alcohol
(D) Isopropyl alcohol
Answer
D
27. In the replacement reaction :
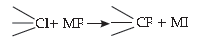
The reaction will be most favourable if M happens to be :
(A) Na
(B) K
(C) Rb
(D) Li
Answer
C
28. HClO4 is a stronger acid than HClO because :
(A) ClO4– ions formed are more stable than ClO– ions.
(B) ClO4– ions formed is less stable than ClO– ions.
(C) Oxygen atoms are less dispersed.
(D) Cl is more electronegative.
Answer
A
29. Which of the given hormone contains iodine :
(A) Insulin
(B) Thyroxine
(C) Adrenaline
(D) Testosterone
Answer
B
30. An organic compound A with molecular formula C6H4O is an isomer of alcohol. When treated with excess HI, it forms two alkyl halides which on hydrolysis gives B and C compounds. Oxidation of B gives an acid D and C gives a mixed ketone. What are D and E ?
(A) Ethanoic acid , Butan-2-one
(B) Propanoic acid, Butan-2one
(C) Butanoic acid , Propan-2-one
(D) Methanoic acid, 3Methyl But-2one
Answer
A
31. Ozone acts as an oxidizing agent due to :
(A) liberation of nascent oxygen
(B) liberation of oxygen gas
(C) both (A) and (B)
(D) none of these
Answer
A
32. Carbon and silicon belong to (IV) group. The maximum coordination number of carbon in commonly occurring compounds is 4, whereas that of silicon is 6. This is due to :
(A) Availability of low lying d-orbitals in silicon
(B) Large size of silicon
(C) More electronegative nature of silicon
(D) Both (B) and (C)
Answer
A
33. Following are the isomeric alcohols with molecular formula C4H9O. Which one will show chirality ?
(A) Pentan-3-ol
(B) 2,2 Dimethylpropan-1-ol.
(C) 3-Methyl butan-2-ol
(D) 2- Methyl butan-2-ol
Answer
C
34. An alkyl halide by formation of its Grignard reagent and heating with water yields propane. What is the original alkyl halide ?
(A) Methyl iodide
(B) Ethyl iodide
(C) ethyl bromide
(D) propyl bromide
Answer
D
35. Which of the following oxidation states are the most characteristic oxidation states for lead and tin respectively ?
(A) +4, +2
(B) +2, +4
(C) +4, +4
(D) +2, +2
Answer
B
36. Which of the following sugar is used as natural sweetener in flavoured water, energy drinks and low calorie products :
(A) Glucose
(B) Galactose
(C) Sucrose
(D) fructose
Answer
D
37. Which of the following statements about H3BO3 is not correct ?
(A) It is a strong tribasic acid.
(B) It is prepared by acidifying an aqueous solution of borax.
(C) It has a layer structure in which planar BO3 units are joined by hydrogen bonds.
(D) It does not act as proton donor but acts as a Lewis acid by accepting hydroxyl ion.
Answer
D
38. Graphite cannot be classified as __________.
(A) Conducting solid
(B) Network solid
(C) Covalent solid
(D) Ionic solid
Answer
D
39. Of the four elements having maximum electron affinity is :
(A) Fluorine
(B) Chlorine
(C) Bromine
(D) Iodine
Answer
B
40. What is the role of water, in the acid catalyzed dehydration of alkene (ethane) ? It acts as :
(A) Electrophile
(B) Nucleophile
(C) Electrophile as well as nucleophile
(D) Free radical
Answer
C
41. Which of the following alcohol does not form a stable compound on dehydration :
(A) methyl alcohol
(B) n-butyl alcohol
(C) n-propyl alcohol
(D) ethyl alcohol
Answer
A
42. Alkene A on reaction with ozone and followed by decomposition with Zn/H2SO4 gives :
(A) alkyl chloride
(B) allyl halide
(C) carbonyl compound
(D) Alkyne
Answer
C
43. The most stable product formed in the above reaction is :
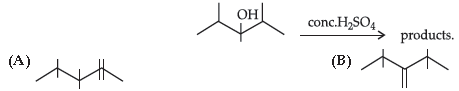
(C) Both (A) and (B)
(D) none of these
Answer
B
44. Which of the following possess highest melting point ?
(A) chlorobenzene
(B) m-dichlorobenzene
(C) o- dichlorobenzene
(D) p- dichlorobenzene
Answer
D
From Q. 45 to Q. 49, Given below are two statements labelled as Assertion (A) and Reason (R) and at the end of each question give the following line select the most appropriate answers from the options given below :
(A) Both A and R are true and R is the correct explanation of A.
(B) Both A and R are true but R is NOT the correct explanation of A.
(C) A is true but R is false.
(D) A is false and R is true.
45. Assertion (A) : F2 has lower bond dissociation energy than Cl2.
Reason (R) : Flourine is more electronegative than chlorine.
Answer
D
46. Assertion (A) : Methoxy ethane reacts with HI to give ethanol and iodomethane.
Reason (R) : Reaction of ether with HI follows SN2 mechanism.
Answer
B
47. Assertion (A) : Vapour pressure increase with increase in temperature.
Reason (R) : With increase in temperature, more molecules of the liquid can go into vapour phase.
Answer
A
48. Assertion (A) : F2 has lower reactivity.
Reason (R) : F-F bond has low Δbond Ho.
Answer
D
49. Assertion (A) : It is safe to inject normal saline solution intravenously.
Reason (R) :The osmotic pressure of a solution is the excess pressure that must be applied to a solutionto prevent osmosis.
Answer
B
Section ‘C’
50. Which is not true statement ?
(A) α-carbon of α-amino acid is asymmetric.
(B) All proteins are found in L-form.
(C) Human body can synthesize all proteins they need.
(D) At pH=7 both amino and carboxylic groups exist in ionized form.
Answer
B
51. What is incorrect about colour of ozone :
(i) It is blue in colour
(ii) It is purple black in solid form
(iii) It is yellow in solid form
(A) Only (i)
(B) Only (iii)
(C) (i) and (ii)
(D) (i) and (iii)
Answer
B
52. Vinyl chloride on reaction with dimethyl copper gives :
(A) Ethyl chloride
(B) Propene
(C) Ethyne
(D) Polyvinyl chloride
Answer
B
CASE 1 : Read the passage given below and answer the following questions 53-55
In an ideal crystal, there must be regular repeating arrangement of the constituting particles and its entropy must be zero at absolute zero temperature. However, it is impossible to obtain an ideal crystal and it suffers from certain defects called imperfections. In pure crystal, these defects arises either due to disorder or dislocation of the constituting particles from their normal positions or due to the movement of the particles even at absolute zero temperature. Such defects increase with rise in temperature. In addition to this, certain defects arise due to the presence of some impurities. Such defects not only modify the existing properties of the crystalline solids but also impart certain new characteristics to them.
53. The solid obtained when AgCl is crystallised from molten AgCl with little of CdCl2 has :
(A) Anionic vacancies
(B) Cationic vacancies equal to double the Cd2+ ions incorporated
(C) Cationic vacancies equal to Cd+2 ions incorporated
(D) Neither of the cationic or ionic vacancies
Answer
C
54. AgI and ZnS shows :
(A) Frenkel defect
(B) Schottky defect
(C) Interstitial defect
(D) Vacancy defect
Answer
A
55. Which stoichiometric defect in crystals increases the density of a solid :
(A) Frenkel defect
(B) Schottky defect
(C) Interstitial defect
(D) Vacancy defect
Answer
C
