Sample Paper Class 9 Science Term 1 Set A
Please refer to Sample Paper Class 9 Science Term 1 Set A with solutions provided below. We have provided CBSE Sample Papers for Class 9 Science as per the latest paper pattern and examination guidelines for Standard 10 Science issued by CBSE for the current academic year. The below provided Sample Guess paper will help you to practice and understand what type of questions can be expected in the Class 9 Science exam.
CBSE Sample Paper Class 9 Science for Term 1 Set A
Question: A 1000 W heater is used for 1 hour everyday for 30 days. Find the cost of electricity if the rate is ` 3.00 per unit.
Answer: The electrical energy consumed = 1000 × 1 × 30
= 3 × 104 Wh = 30 kWh = 30 units
Question: Suppose gravity of Earth suddenly becomes zero, then in which direction will the Moon begin to move if no other celestial body affects it?
Answer: The Moon will not move in the direction of the tangent to the Moon’s orbit.
Question: Which one of the following pairs of gases contains the same number of molecules?
(a) 16 g of O2 and 14 g of N2
(b) 8 g of O2 and 22 g of CO2
(c) 28 g of N2 and 22 g of CO2
(d) 32 g of O2 and 32 g of N2
Answer: A
Question: Work is said to be done if the force and displacement are
(a) parallel to each other
(b) opposite to each other
(c) inclined at an angle with each other (q ≠ 90°)
(d) all of the above.
Answer: D
OR
Question: The energy possessed by an aeroplane flying at an altitude is
(a) elastic potential energy
(b) kinetic energy
(c) mechanical energy
(d) gravitational potential energy.
Answer: C
Question: In vaccum, all freely falling objects
(a) have the same speed
(b) have the same velocity
(c) have the same acceleration
(d) have the same force.
Answer: C
Question: Let M denote the mass of Earth and let R denote its radius. The ratio g/G at Earth’s surface is
(a) R2/M
(b) M/R2
(c) M/R
(d) R/M
Answer: B
For question numbers 6 and 7, two statements are given- one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below :
(a) Both A and R are true, and R is correct explanation of the assertion.
(b) Both A and R are true, but R is not the correct explanation of the assertion.
(c) A is true, but R is false.
(d) A is false, but R is true.
Question: Assertion : Percentage of carbon in Na2CO3 is 11.32%.
Reason : % of Carbon= Mass of carbon element/Molecular mass of Na2CO3×100
Answer: A
Question: Assertion : Majority of children in many parts of India are already immune to hepatitis A by the time they are five years old.
Reason : Children are exposed to the virus through water.
OR
Question: Assertion : Rabies virus is spread by the bite of infected dogs and other animals.
Reason : There are anti-rabies vaccines for both humans and animals.
Answer: B
Read the following and answer any four questions from 8(i) to 8(v).
The composition of protons, electrons and neutrons in an atom can be determined from it’s atomic number and mass number. Atomic number or protons number of an element is equal to the number of protons present in the nucleus of an atom of that element. Atomic number is always a whole number.
Question: In the nucleus of 4020Ca, there are
(a) 40 protons and 20 electrons
(b) 20 protons and 40 electrons
(c) 20 protons and 20 neutrons
(d) 20 protons and 40 neutrons.
Answer: C
Question: The number of neutrons in the element 94Be is
(a) 4
(b) 5
(c) 9
(d) 13
Answer: B
Question: How many total protons are found in one molecule of retinol (C20H30O)?
(a) 51
(b) 151
(c) 600
(d) 158
Answer: D
Question: If there are 12 neutrons in an atom and its atomic number is 11 then how many electrons are present in it?
(a) 23
(b) 12
(c) 10
(d) 11
Answer: D
Question: The nucleon number of atom X is 37. It exists as a diatomic molecule, X2. One molecule of X2 contains 34 protons. How many neutrons are present in the nucleus of atom X?
(a) 17
(b) 20
(c) 21
(d) 25
Answer: B
Question: Refer to the given figures and answer any four questions from 9(i) to 9(v). image
(i) The given organism A is
(a) HIV
(b) Leishmania
(c) Helicobacter pylori
(d) Penicillium.
Answer: B
Question: Select the correct statement(s) regarding the organism A.
(i) The organism shown is a protozoa.
(ii) This organism causes peptic ulcers.
(iii) This organism can be extracted from the small intestine.
(iv) This organism can be transmitted from person to person through housefly.
(a) (ii) and (iii)
(b) Only (i)
(c) (i) and (iv)
(d) (i), (ii) and (iii)
Answer: B
Question: The organism B is responsible for causing
(a) sleeping sickness
(b) ascariasis
(c) AIDS
(d) kala-azar.
Answer: B
Question: The organism B is found in the _______ of infected persons.
(a) eyes
(b) internal ears
(c) large intestine
(d) small intestine
Answer: D
Question: Which of the organisms A and B does not require a secondary host to complete its life cycle?
(a) Organism A
(b) Organism B
(c) Both (a) and
(b) (d) None of these
Answer: B
SECTION – b
Question: Calculate the number of moles in 12.044 × 1023 helium atoms.
Answer: 6.022 × 1023 helium atoms = 1 mole
12.044 × 1023 helium atoms
= 1/6.022x 1023x 12.044×1023=2 moles
Question: The atom of an element has 2 electrons in the M-shell. What will be the atomic number of the element? Name the element.
OR
Lithium atom has atomic mass 7 and has 3 protons in it nucleus. How many neutrons does it have? Write down its electronic configuration.
Answer: As the atom has 2 electrons in the M-shell, this means that K and L-shells are completely filled. As completely filled K-shell has 2 electrons and completely filled L-shell has 8 electrons, therefore, complete electronic configuration of the atom of the element will be
K L M
2 8 2
∴ Total number of electrons = 2 + 8 + 2 = 12
As the atom is neutral, total number of protons = total number of electrons = atomic number = 12. Hence, the element is magnesium.
OR
Mass number (A) of lithium = Atomic mass = 7
No. of protons in the nucleus = Atomic number (Z) = 3
No. of neutrons (n) = A – Z = 7 – 3 = 4
Its electronic configuration is K L
2 1 .
Question: Newton’s law of gravitation is also called inverse-square law. Why is it so called?
Answer: According to Newton’s law of gravitation, the magnitude of the gravitational force between two objects is inversely proportional to the square of the distance between them, i.e., F ∝ 1/r2. That is why this law is also called inverse-square law.
SECTION – C
Question: Why making anti-viral medicines is harder than making anti-bacterial medicines?
OR
What is an antibiotic ? Describe the mechanism of action of antibiotics with the help of an example.
Answer: Viruses lack any cell wall of their own. They enter the host cell and use its mechanism for their life processes.
They do not have cytoplasm and cell organelles. To multiply they enter the cells of living organisms. They use cellular resources of the host to reproduce. As viruses lack cells, biochemical processes are missing in them. Since these biochemical processes are the targets of antibiotics, antibiotics do not work on viruses.
OR
Antibiotics are the chemicals secreted by microorganisms like fungi and bacteria that kill or hinder the growth of certain other kinds of microbes like bacteria. E.g., penicillin.
Antibiotics commonly block biochemical pathways important for bacteria. Many bacteria make a cell wall to protect themselves. The antibiotic penicillin blocks the biochemical processes that build the cell wall.
Consequently, the growing bacteria become unable to make cell walls and die easily.
Question: Two objects of masses m1 and m2 having the same size are dropped simultaneously from heights h1 and h2respectively. Find out the ratio of time they would take in reaching the ground.
Answer: Using the formula, s = ut + 1/2 at 2, we can write

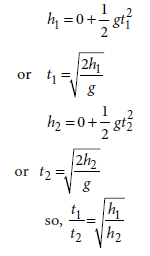
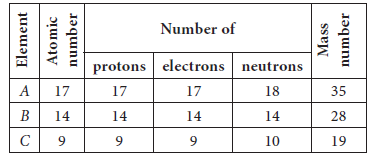
Question: Complete the following table. Answer: For a neutral atom, we know that,
Atomic number = No. of protons
No. of electrons = No. of proton = Atomic number
Mass number = No. of protons + No. of neutrons
SECTION – D
Question: (i) Drive an expression for potential energy of a body of mass m, at a height h above the surface of the earth.
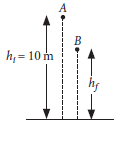
(ii) A ball is dropped from a height of 10 m. If the energy of the ball reduced by 40% after striking the ground, how much high can the ball bounce back ?
OR
(i) Define the work done by a constant force. Write its SI unit and define this unit.
(ii) A 3000 kg truck moving at a speed of 90 m/s stops after covering some distance. The force applied by brakes is 27000 N. Compute the distance covered and work done by this force.
Answer: (i) We know that every object on the surface of the Earth is attracted by the Earth.
If an object has a mass m, it will experience a force that is called the weight (W = mg) of the object which acts vertically downwards.
For all practical purposes, we assume that the value of g remains
constant near the surface of the Earth, so that the weight of the object remains constant.
To move this object upwards from A to B through a distance h, we must apply a force F upwards where F = mg as shown in figure. Work done by force F, i.e.,W = F h
But as F = mg, therefore W = mgh …(i)
This work done on the object which changes its position is called the gravitational potential energy. Further, this work does not go waste and instead is stored in the object in the form of its potential energy. If the potential energy of the body is denoted by Ep, then Ep = W …(ii) From eqns. (i) and (ii), we get Ep = mgh

(ii) Let the ball be dropped from the point A and after bouncing back it reached the point B. It loses 40% energy i.e., now at point B it possesses 60% of energy.
Potential energy at point B = 60% of potential energy at point A.
∴ mghf = 0.6 × mghi
Or hf = 0.6 × hi = 0.6 × 10 = 6 m
OR
(i) Work is said to be done when a force acts on an object and the object displaced by some distance.
Its SI unit is Joule.
One joule : When a force of 1 N displaces a body through 1 m in the direction of force.
(ii) Velocity, u = 90 m/s, F = –27000 N, m = 3000 kg As, F = ma
or a = F/m = –27000/3000 = –9 m/s2
Also, v2 – u2 = 2as ⇒ 02 – (90)2 = 2(–9) s
∴ s = 450 m
Now, W = F × s
= – 27000 × 450 = –12150000 J = – 12150 kJ
Negative sign shows retarding force.
Question: Calculate the percentage by mass of each element in sodium thiosulphate, Na2S2O3.
OR
Calculate the number of moles of phosphorus (P) atoms in 100 g of phosphorus. If phosphorus is considered to contain P4 molecules, then how many moles of P4 molecules are there?
Answer:



