MCQs for Physics Class 11 with Answers Chapter 12 Thermodynamics
Students of class 11 Physics should refer to MCQ Questions Class 11 Physics Thermodynamics with answers provided here which is an important chapter in Class 11 Physics NCERT textbook. These MCQ for Class 11 Physics with Answers have been prepared based on the latest CBSE and NCERT syllabus and examination guidelines for Class 11 Physics. The following MCQs can help you to practice and get better marks in the upcoming class 11 Physics examination
Chapter 12 Thermodynamics MCQ with Answers Class 11 Physics
MCQ Questions Class 11 Physics Thermodynamics provided below have been prepared by expert teachers of grade 11. These objective questions with solutions are expected to come in the upcoming Standard 11 examinations. Learn the below provided MCQ questions to get better marks in examinations.
Question. If the amount of heat given to a system is 35 J and the amount of work done on the system is 15 J, then the change in internal energy of the system is
(a) –50 J
(b) 20 J
(c) 30 J
(d) 50 J
Answer
D
Question. An ideal gas having molar specific heat capacity at constant volume is 3/2 R , the molar specific heat capacities at constant pressure is
(a) 1/2 R
(b) 5/2 R
(c) 7/2 R
(d) 9/2 R
Answer
B
Question. An ideal gas undergoes cyclic process ABCDA as shown in given P-V diagram. The amount of work done by the gas is
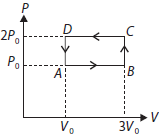
(a) 6P0V0
(b) –2P0V0
(c) +2P0V0
(d) +4P0V0
Answer
B
Question. The relation between the slope of isothermal curve and slope of adiabatic curve
(a) slope of adiabatic curve = ϒ times slope of isothermal curve
(b) slope of isothermal curve = ϒ times slope of adiabatic curve
(c) slope of adiabatic curve = ϒ2 times slope of isothermal curve
(d) slope of isothermal curve = ϒ2 times slope of adiabatic curve
Answer
A
Question. During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio CP/CV for gas is
(a) 4/3
(b) 2
(c) 5/3
(d) 3/2
Answer
D
Question. Consider the process on a system shown in figure below. During the process, the work done by the system
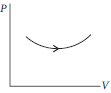
(a) continuously increases
(b) continuously decreases
(c) first increases then decreases
(d) first decreases then increases
Answer
A
Question. A thermodynamic process of one mole of an monoatomic ideal gas is shown in the figure. The efficiency of cyclic process ABCA will be
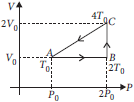
(a) 25%
(b) 12.5%
(c) 50%
(d) 7.7%.
Answer
D
Question. One mole of an ideal gas goes from an initial state A to final state B via two processes : It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct P-V diagram representing the two processes is
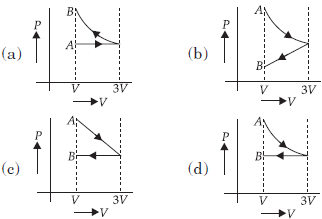
Answer
D
Question. For a diatomic gas change in internal energy for unit change in temperature for constant pressure and constant volume is ΔU1 and ΔU2 respectively. The ratio of ΔU1 : ΔU2 is
(a) 5 : 3
(b) 3 : 5
(c) 1 : 1
(d) 5 : 7
Answer
C
Question. A thermodynamic system is taken through the cycle ABCD as shown in figure. Heat rejected by the gas during the cycle is
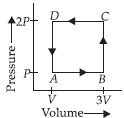
(a) 2 PV
(b) 4 PV
(c) 1/2 PV
(d) PV
Answer
A
Question. Which one of the following graphs represents variation of specific heat capacity of water with temperature?
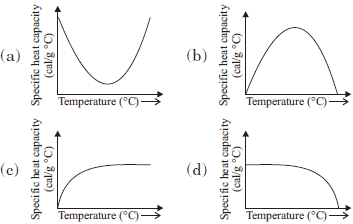
Answer
A
Question. One mole of an ideal gas is taken from A to B, from B to C and then back to A. The variation of its volume with temperature for that change is as shown. Its pressure at A is P0, volume is V0. Then, the internal energy

(a) at A is more than at B
(b) at C is less than at B
(c) at B is more than at A
(d) at A and B are equal
Answer
D
Question. A thermodynamic process is shown in figure. In process ab, 600 J of heat is added, and in process bd 200 J of heat is added. The total heat added in process acd is
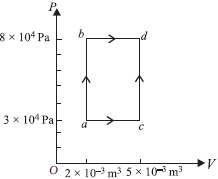
(a) 550 J
(b) 650 J
(c) 750 J
(d) 850 J
Answer
B
Question. An ideal gas is taken through the cycle A → B → C → A, as shown in figure. If the net heat supplied to the gas in the cycle is 5 J, the work done by the gas in the process C → A is
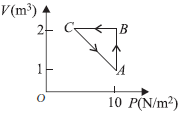
(a) – 5 J
(b) – 10 J
(c) – 15 J
(d) – 20 J
Answer
A
Question. 1 mole of an ideal gas in a cylindrical container have the P-V diagram as shown in figure. If V2 = 4V1, then the ratio of temperatures T1/T2 will be

(a) 1/2
(b) 1/4
(c) 3/2
(d) 3/4
Answer
A
Question. For an ideal gas, the equation of a process for which the heat capacity of the gas varies with temperature as C = α/T (a is a constant) is given by
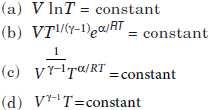
Answer
B
Question. An ideal gas is taken around the cycle ABCA as shown in the P-V diagram. The total work done by the gas during the cycle is
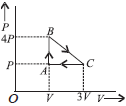
(a) PV
(b) 2PV
(c) 4PV
(d) 3PV
Answer
D
Question. The internal energy of a gas in an adiabatic process is given by U = a + bPV, find ϒ.

Answer
B
Question. Consider two containers A and B containing identical gases at the same pressure, volume and temperature. The gas in container A is compressed to half of its original volume isothermally while the gas in container B is compressed to half of its original value adiabatically. The ratio of final pressure of gas in B to that of gas in A is

Answer
A
Question. One mole of an ideal gas is taken through a cyclic process as shown in the V-T diagram. Which of the following statements is true?
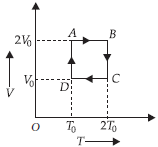
(a) The magnitude of work done by the gas is RT0ln2.
(b) Work done by gas is V0T0.
(c) Net work done by the gas is zero.
(d) Work done by the gas is 2RT0ln2.
Answer
A
Assertion & Reasoning Based MCQs :
Assertion (A) and the other labelled
Reason (R) Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below.
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true but R is NOT the correct explanation of A
(c) A is true but R is false
(d) A is false and R is also false
Question. Assertion (A) : An adiabatic process is an isoentropic process.
Reason (R) : Change in entropy is zero in case of adiabatic process.
Answer
A
Question. Assertion (A) : First law of thermodynamics is a restatement of the principle of conservation of energy.
Reason (R) : Energy is a fundamental quantity.
Answer
C
Question. Assertion (A) : Two systems in thermal equilibrium with a third system, are in thermal equilibrium with each other.
Reason (R) : Heat flows spontaneously from a system at a higher temperature to a system at a lower temperature.
Answer
B
Question. Assertion (A) : In an adiabatic compression, the internal energy and temperature of the system get decreased.
Reason (R) : An adiabatic compression is a slow process.
Answer
C
Question. Assertion (A) : We can change the temperature of a body without giving (or taking) heat to (or from) it.
Reason (R) : According to principle of conservation of energy, total energy of a system should remain conserved.
Answer
B
Question. Assertion (A) : In an adiabatic process, change in internal energy of a gas is equal to work done on or by the gas in the process.
Reason (R) : Temperature of gas remains constant in an adiabatic process.
Answer
C
Question. Assertion (A) : Work done by a gas in isothermal expansion is more than the work done by the gas in the same expansion, adiabatically.
Reason (R) : Temperature remains constant in isothermal expansion and not in adiabatic expansion.
Answer
B
Question. Assertion (A) : The internal energy of a real gas is function of both, temperature and volume.
Reason (R) : Internal kinetic energy depends on temperature and internal potential energy depends on volume.
Answer
A
Question. Assertion (A) : A gas does not have a unique value of specific heat.
Reason (R) : Specific heat is defined as the amount of heat required to raise the temperature of unit mass of the substance through unit degree.
Answer
B
Question. Assertion (A) : The internal energy of an isothermal process does not change.
Reason (R) : The internal energy of a system depends only on pressure of the system.
Answer
C
Case Based MCQs :
First Law of Thermodynamics
The first law of thermodynamics is the general law of conservation of energy applied to any system in which energy transfer from or to the surroundings (through heat and work) is taken into account. It states that the energy supplied to the system goes in partly to increase the internal energy of the system and the rest in work on the environment.
Mathematically, ΔQ = ΔU + ΔW
where ΔQ is the heat supplied to the system, ΔW is the work done by the system and ΔU is the change in internal energy of the system. ΔQ and ΔW depend on the path taken to go from initial to final states, but the combination ΔQ – ΔW is path independent.
Question. Which of the following is not a path function ?
(a) DQ
(b) DQ + DW
(c) DW
(d) DQ – DW
Answer
D
Question. A certain mass of gas is carried from A to B, along three paths via ACB, ADB and AEB. Which one of the following is correct?
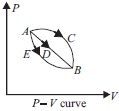
(a) Work done via path ACB is minimum.
(b) Work done via path ADB is maximum.
(c) Work done via path ACB is maximum.
(d) Work done via path AEB is maximum.
Answer
C
Question. An electric heater supplies heat to a system at a rate of 120 W. If system performs work at a rate of 80 J s–1, the rate of increase in internal energy is
(a) 30 J s–1
(b) 40 J s–1
(c) 50 J s–1
(d) 60 J s–1
Answer
B
Question. A system goes from A to B by two different paths in the P-V diagram as shown in figure. Heat given to the system in path 1 is 1100 J, the work done by the system along path 1 is more than path 2 by 150 J. The heat exchanged by the system in path 2 is
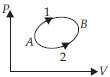
(a) 800 J
(b) 750 J
(c) 1050 J
(d) 950 J
Answer
D
Question. The first law of thermodynamics is concerned with conservation of
(a) number of molecules
(b) number of moles
(c) energy
(d) temperature
Answer
C
Thermodynamic Process
Isothermal process : A thermodynamic process in which the temperature remains constant.
Equation of isothermal process, PV = constant.
Adiabatic process : A thermodynamic process in which no heat flows between the system and the surroundings.
Equation of adiabatic process, PVg = constant where g = CP/CV. If an ideal gas undergoes a change in its state adiabatically from (P1, V1) to (P2, V2).
P1, V1ϒ = P2, V2ϒ
Question. An ideal gas A and a real gas B have their volumes increased from V to 2V under isothermal conditions The increase in internal energy
(a) will be same in both A and B
(b) will be zero in both the gases
(c) of B will be more than that of A
(d) of A will be more than that of B
Answer
B
Question. A thermoflask made of stainless steel contains several tiny lead shots. If the flask is quickly shaken up and down several times, the temperature of lead shots
(a) increases by adiabatic process
(b) increases by isothermal process
(c) decreases by adiabatic process
(d) remains same
Answer
A
Question. For an ideal gas, in an isothermal process,
(a) heat content remains constant.
(b) heat content and temperature remains constant
(c) temperature remains constant
(d) heat energy varies.
Answer
C
Question. If an ideal gas is compressed isothermally, then
(a) no work is done against gas
(b) heat is released by the gas.
(c) the internal energy of gas will increase
(d) pressure does not change.
Answer
B
Question. No heat flows between the system and surrounding. Then the thermodynamic process is
(a) isothermal
(b) isochoric
(c) adiabatic
(d) isobaric
Answer
C

