MCQs for Science Class 10 with Answers Chapter 1 Chemical Reactions and Equations
Students of class 10 Science should refer to MCQ Questions Class 10 Science Chemical Reactions and Equations with answers provided here which is an important chapter in Class 10 Science NCERT textbook. These MCQ for Class 10 Science with Answers have been prepared based on the latest CBSE and NCERT syllabus and examination guidelines for Class 10 Science. The following MCQs can help you to practice and get better marks in the upcoming class 10 Science examination
Chapter 1 Chemical Reactions and Equations MCQ with Answers Class 10 Science
MCQ Questions Class 10 Science Chemical Reactions and Equations provided below have been prepared by expert teachers of grade 10. These objective questions with solutions are expected to come in the upcoming Standard 10 examinations. Learn the below provided MCQ questions to get better marks in examinations.
Question. A reddish brown coloured metal used in electric wires, when powdered and heated strongly in an open China dish, its colour turns black. When hydrogen gas is passed over this black substances, it regain its original colour. Based on this information, the metal and black coloured substances are
(a) copper and copper nitrate
(b) silver and silver oxide
(c) copper and copper oxide
(d) aluminium and aluminium oxide
Answer
C
Question. In the equation, NaOH + HNO3 → NaNO3 + H2O nitric acid is acting as-
(a) an oxidising agent
(b) an acid
(c) a nitrating agent
(d) a dehydrating agent
Answer
B
Question. CuO + H2 → H2O + Cu , reaction is an example of –
(a) redox reaction
(b) synthesis reaction
(c) neutralisation
(d) analysis reaction
Answer
A
Question. Zn + H2SO4(dil) → ZnSO4 + H2↑
Above reaction is –
(a) decomposition reaction
(b) single displacement reaction
(c) combination reaction
(d) synthesis reaction
Answer
B
Question. Which one of the following involve a chemical reaction?
(a) Evaporation of water
(b) Storing on nitrogen gas under pressure
(c) Keeping petrol in a China dish in open
(d) Heating magnesium wire in the presence of air at high temperature
Answer
D
Question. The reaction in which two compounds exchange their ions to form two new compounds is – (a) a displacement reaction
(b) a decomposition reaction
(c) an isomerization reaction
(d) a double displacement reaction
Answer
D
Question. When dilute sulphuric acid is added to pieces of iron sulphide, hydrogen sulphide gas is produced and soluble ferrous sulphate is formed. The type of chemical reaction involved is
(a) decomposition reaction
(b) combination reaction
(c) displacement reaction
(d) double displacement reaction
Answer
D
Question. A redox reaction is one in which-
(a) both the substance are reduced
(b) both the substance are oxidised
(c) an acid is neutralised by the base
(d) one substance is oxidised while the other is reduced
Answer
D
Question. Combination of phosphorus and oxygen is an example of –
(a) oxidation
(b) reduction
(c) rancidity
(d) None of these
Answer
A
Question. When the gases sulphur dioxide and hydrogen sulphide mix in the presence of water, the reaction is SO2 + 2H2S → 2H2O + 3S . Here hydrogen sulphide is acting as –
(a) an oxidising agent
(b) a reducing agent
(c) a dehydrating agent
(d) a catalyst
Answer
B
Question. In the following equations :
Na2CO3 + x HCl → 2NaCl + CO2 + H2O , the value of x is-
(a) 1
(b) 2
(c) 3
(d) 4
Answer
B
Question. To indicate the presence of gaseous reactant or product, we use the symbol
(a) (Product)g or (Reactant)g
(b) (Product)- or (Reactant)-
(c) (Product). or (Reactant).
(d) Both (a) and (b)
Answer
D
Question. Which of the following is an endothermic process?
(a) Dilution of sulphuric acid
(b) Sublimation of dry ice
(c) Condensation of water vapours
(d) Respiration in human beings
Answer
B
Question. The condition produced by aerial oxidation of fats and oils in foods marked by unpleasant smell and taste is called:
(a) Antioxidation
(b) Reduction
(c) Rancidity
(d) Corrosion
Answer
C
Question. Which of the following reactions will not take place?
(a) Zn + CuSO 4 → ZnSO 3 + Cu
(b) 2KBr + Cl 2 →KCI+ Br 2
(c) Zn + MgSO 4 → ZnSO 4 + Mg
(d) Mg + FeSO 4 – MgSO 4 + Fe
Answer
C
Question. The reaction between lead nitrate and potassium iodide present in aqueous solutions is an example of
(a) Decomposition Reaction
(b) Displacement Reaction
(c) Double Displacement Reaction
(d) Neutralisation Reaction
Answer
C
Question. What type of chemical reactions take place when electricity is passed through water?
(a) Displacement
(b) Combination
(c) Decomposition
(d) Double displacement
Answer
C
Question. Give the ratio in which hydrogen and oxygen are present in water by volume.
(a) 1:2
(b) 1:1
(c) 2:1
(d) 1:8
Answer
A
Question. A substance which oxidizes itself and reduces other is known as
(a) Oxidising agent
(b) reducing agent
(c) Both (a) and (b)
(d) None of these.
Answer
B
Question. Oxidation is a process which involves
(a) addition of oxygen
(b) addition of hydrogen
(c) removal of oxygen
(d) removal of hydrogen
Answer
A
Question. Pb + CuCl2 → PbCl2 + Cu The above reaction is an example of:
(a) combination
(b) double displacement
(c) decomposition
(d) displacement
Answer
D
Question. In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
(a) 2H2 (l) + O2 (l) → 2H2O (g)
(b) 2H2 (g) + O2 (l) → 2H2O (l)
(c) 2H2 (g) + O2 (g) → 2H2O (l)
(d) 2H2 (g) + O2 (g) → 2H2O (g)
Answer
C
Question. Which of the following gases can be used for storage of fresh sample of an oil for a long time?
(a) Carbon dioxide or Oxygen
(b) Nitrogen or Oxygen
(c) Carbon dioxide or Helium
(d) Helium or Nitrogen
Answer
D
Question. Name the products formed when iron filings are heated with dilute hydrochloric acid
(a) Fe (III) chloride and water
(b) Fe (II) chloride and water
(c) Fe (II) chloride and hydrogen gas
(d) Fe (III) chloride and hydrogen gas
Answer
D
Question. A dilute ferrous sulphate solution was gradually added to the beaker containing acidified permanganate solution. The light purple colour of the solution fades and finally disappears. Which of the following is the correct explanation for the observation?
(a) KMnO4 is an oxidising agent, it oxidises FeSO4.
(b) FeSO4 acts as an oxidising agent and oxidises KMNO4.
(c) The colour disappears due to dilution; no reaction is involved.
(d) KMnO4 is an unstable compound and decomposes in presence of FeSO4 to a colourless compound.
Answer
A
Question. When green coloured ferrous sulphate crystals are heated, the colour of the crystal changes because
(a) it is decomposed to ferric oxide
(b) it loses water of crystallisation
(c) it forms SO2
(d) it forms SO3
Answer
B
Question. In an electrolytic cell where electrolysis is carried, anode has:
(a) Positive change
(b) Negative charge
(c) Connected to negative terminal of the battery
(d) None of these is correct
Answer
A
Question. Lead nitrate Pb(NO3)2 on heating forms solid Lead oxide (PLO) and Nitrogen direide What is the colour of lead oxide and nitrogen dioxide?
(a) White, Colourless
(b) White, Brown
(c) Yellow. Brown
(d) Yellow. Colourless
Answer
C
Question. Which law should be kept in mind while we balance chemical equations?
(a) Conservation of momentum
(b) Conservation of mass
(c) Conservation of energy
(d) Conservation of frequency
Answer
B
Question. The oxidation reaction which produces heat and light is called
(a) Endothermic
(b) Photochemical
(c) Exothermic
(d) Combustion
Answer
D
Question. Any reaction that produces a precipitates can be called a………………………….. reaction.
(a) Displacement reaction
(b) Combination reaction
(c) Precipitates reaction
(d) Exothermic reaction
Answer
C
Question. What does the symbol “↓” represent in a chemical reaction?
(a) Gas released
(b) Solid state
(c) Precipitate formed
(d) Direction of reaction
Answer
C
Question. Which of the following is an endothermic reaction ?
(a) Burning of coal
(b) Respiration
(c) Decomposition of vegetable matter into compost
(d) Decomposition of calcium carbonate to form quick lime and carbon dioxide
Answer
D
Question. Formula of Slaked lime or Calcium hydroxide is
(a) CaO
(b) Ca(OH)2
(c) C6H12O6
(d) CaCO3
Answer
B
Question. A white precipitate will be formed if we add common salt solution to
(a) Ba(NO3)2 solution
(b) KNO3 solution
(c) AgNO3 solution
(d) Mg(NO3)2 solution
Answer
C
Question. A slice of apple acquires a brown colour due to :-
(a) Physical change
(b) Chemical change
(c) Natural change
(d) All of these
Answer
B
Question. Which of the following gases can be used for storage of fresh samples of an oil for a long time?
(a) Carbon dioxide
(b) Nitrogen
(c) Hydrogen
(d) Helium
Answer
D
Question. Pb + CuCl2 → PbCl2 + Cu The above reaction is an example of:
(a) combination
(b) double displacement
(c) decomposition
(d) displacement
Answer
D
Question. Which of the following observation belp(s) us to determine that a chemical change has taken place?
(a) Change in temperature.
(b) Change in color
(c) Evolution of a gas.
(d) All of these
Answer
D
Question. Which method can be used to prevent rancidity ?
(a) Coating of paint
(b) Galvanization
(c) Use of Refrigerator
(d) Use of Grease or oil
Answer
C
Question. Which Among The Following (Are) Double Displacement Reaction (S)?
(a) Pb + CuCl2 → PbCl2 + Cu, and CH4 + 2O2 → CO2 + 2H2O
(b) Na2SO4 + BaCl2 → BaSO4 + 2NaCl
(c) Pb + CuCl2 → PbCl2 + Cu, and Na2SO4 + BaCl2 → BaSO4 + 2NaCl
(d) C + O2 → CO2, and CH4 + 2O2 → CO2 + 2H2O
Answer
B
Question. Metals attacks by substances around such as moisture, acid that we called metal get……..
(a) corroded
(b) rusting
(c) moisturize
(d) evaporated
Answer
A
Question. Copper displaces which of the following metals from its salt solution:
(a) ZnSO 4
(b) FeSO 4
(c) AgNO 3
(d) NiSO 4
Answer
C
Question. Which of the following involves combination of two elements?
(a) N 2 (g) + 3H 2(g) + 2NH 3 (g)
(b) Cao(s) + CO 2 (g) → CaCO 3 (g)
(c) 2SO 2 (g) + O 2 (g) → 2SO 3 f(g)
(d) NH 3 (g) + HCl(g) – NH 4 CI(S)
Answer
A
Question. Some crystals of copper sulphate were dissolved in water. The colour of the solution obtained would be:
(a) green
(b) red
(c) blue
(d) brown
Answer
C
Question. When dilute HCl is added to zinc pieces taken in a test tube
(a) No change takes place
(b) the colour of the solution becomes yellow.
(c) A pungent smelling gas gets liberated.
(d) small bubbles of H 2 gas appear on the surface of zinc pieces
Answer
D
Question. Pb + CuCl 2 → PbCl 2 + Cu This reaction is an example of:
(a) combination
(b) displacement
(c) decomposition
(d) double displacement
Answer
B
Question. Magnesium ribbon is rubbed before burning because it has a coating of:
(a) basic magnesium oxide
(b) basic magnesium carbonate
(c) basic magnesium sulphide
(d) basic magnesium chloride
Answer
B
Question. Which of the following reactions is not correct:
(a) Zn+ CuSO 4 → ZnSO 4 + Cu
(b) 2Ag + Cu(NO3 ) 2 → 2AgNO 3 + Cu
(c) Fe+CuSO 4 → FeSO 4 + Cu
(d) Mg + 2HCl → MgCl 2 + H 2
Answer
B
Question. Which one of the following processes involve chemical reactions?
(a) Storing of oxygen gas under pressure in a gas cylinder
(b) Liquefaction of air
(c) Keeping petrol in a china dish in the open
(d) Heating copper wire in presence of air at high temperature
Answer
D
Question. Assertion: MnO 2 + 4HCl → MnCl 2 + Cl 2 + 2H 2 O is redox reaction.Reason: MnO 2 oxides HCl to Cl 2 and gets reduced to MnCl.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
(e) Both A and R are false.
Answer
A
Question. Heat is evolved during:
(a) Endothermic Reaction
(b) Displacement Reaction
(c) Combustion Reaction
(d) Combination Reaction
Answer
C
Question. Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is:
(a) 1:1
(b) 2:1
(c) 4:1
(d) 1:2
Answer
B
Question . Dissolving suger is an example of
(a) Physical change
(b) Chemical change
(c) Redox Reaction
(d) None of these.
Answer
A
Question. What is the name of substance which does get deposited over iron because of moisture present in air?
(a) Sulphide
(b) Rust
(c) Carbonate
(d) Oxygen
Answer
B
Question. A researcher adds barium hydroxide to hydrochloric acid to form a white coloured barium chloride. Which option gives the balanced chemical equation of the reaction?
(a) HCl + 2Ba(OH) → 2BaCl2 + 2H2O + O2
(b) 2HCl + Ba(OH)2 → BaCl2 + 2H2O
(c) 2HCl + Ba(OH)2 → BaH2 + 2HCl + O2
(d) 2HCl + Ba(OH)2 → BaH2 + 2HCl + O2
Answer
B
Question. A student learns that some products are formed as a result of combining two compounds while some products are formed as a result of dissociation of two compounds. Two reactions are:
Reaction P – CaO + SO3 → CaSO4
Reaction Q – ZnCO3 → ZnO + CO2
Which reaction is an example of a combination reaction and a decomposition reaction?
(a) Both the reactions are examples of a decomposition reaction.
(b) P is an example of a decomposition reaction while reaction Q is an example of a combination reaction.
(c) Reaction P is an example of a combination reaction while reaction Q is an example of a decomposition reaction.
(d) Both the reactions are examples of combination reaction.
Answer
C
Question. Why magnesium ribbon is cleaned before burning?
(a) To remove dust
(b) To remove magnesium oxide
(c) To remove magnesium
(d) All of the above
Answer
B
Question. What happens when hydrogen reacts with oxygen?
(a) Carbon dioxide is formed
(b) Water is formed
(c) Hydrogen carbonate is formed
(d) All of the above
Answer
B
Question. Which of the following product is formed when calcium oxide reacts with water?
(a) Slaked lime
(b) Carbon dioxide
(c) Calcium oxide
(d) Oxygen gas
Answer
A
Question. Heating of ferrous sulphate gives which of the following product?
(a) Ferric oxide
(b) Sulphur dioxide
(c) Sulphur trioxide
(d) All of the above
Answer
D
Question. In which of the following category will you put the reaction of heating of ferrous sulphate?
(a) Decomposition reaction
(b) Combination reaction
(c) Displacement reaction
(d) All of the above
Answer
A
Question. What happens when silver chloride is put under sunlight?
(a) Silver metal and chlorine gas are formed
(b) Silver metal and hydrogen gas are formed
(c) Only silver metal is formed
(d) Only hydrogen gas is formed
Answer
A
Question. Which of the following is formed when lead nitrate is put under thermal decomposition?
(a) Lead oxide
(b) Nitrogen dioxide
(c) Oxygen gas
(d) All of the above
Answer
D
Question. What happens when carbon dioxide is passed through lime water?
(a) Lime water turns milky because of formation of calcium carbonate
(b) Lime water turns milky because of formation of water
(c) Lime water turns red because of formation of permanganate
(d) Lime water turns red because of formation of copper sulphate
Answer
A
Question. What happens when silver bromide is exposed to sunlight?
(a) Hydrogen gas is formed
(b) Bromine gas is formed
(c) Chlorine gas is formed
(d) Iodine gas is formed
Answer
B
Question. Which metal is displaced when lead is put in the solution of copper chloride?
(a) Lead
(b) Copper
(c) Chlorine
(d) All of the above.
Answer
B
Question. Which of the following is formed when lead metal reacts with the solution of copper chloride?
(a) No reaction takes place
(b) Chlorine gas
(c) Lead chloride
(d) Copper-lead complex
Answer
C
Question. Which metal is displaced when zinc metal is put in the solution of copper sulphate?
(a) Copper
(b) Zinc
(c) Sulphate
(d) All of the above
Answer
A
Question. Which of the following is a displacement reaction?
(a) MgCO3 → MgO + CO2’
(b) 2Na + 2H2O → 2NaOH + H2
(c) 2H2 + O2 → 2H2O
(d) 2Pb (NO3)2 → 2PbO + 4NO2 + O2
Answer
B
Question. A substance ‘X’ is used in white-washing and is obtained by heating limestone in the absence of air. The substance ‘X’ is
(a) CaOCl2
(b) Ca(OH)2
(c) CaO
(d) CaCO3
Answer
C
Question. Identify ‘x’, ‘y’ and ‘z’ in the following reaction:
2KClO3(x) →(y) 2KCl(x) + O2(z)
(a) x = gas; y = reaction condition, z = gas
(b) x = solid; y = liquid; z = gas
(c) x = number of moles of KClO3; y = reaction condition; z = no. of molecules of oxygen.
(d) x = physical state of KClO3 and KCl; y = reaction condition; z = physical state of O2.
Answer
D
Question. In which of the following the identity of initial substance remains unchanged?
(a) Curdling of milk
(b) Formation of crystals by process of crystallisation
(c) Fermentation of grapes
(d) Digestion of food
Answer
B
Question. What happens when sodium sulphate solution is mixed with the solution of barium chloride?
(a) Barium sulphate is formed
(b) Sodium sulphate is formed
(c) Sulphur dioxide gas is formed
(d) No reaction takes place
Answer
A
Question. What happens when copper metal is dipped in the solution of zinc sulphate?
(a) Copper sulphate is formed
(b) Oxygen gas is formed
(c) Zinc metal is separated
(d) No reaction takes place
Answer
D
Question. What happens when zinc metal is dipped in the solution of copper sulphate?
(a) Zinc sulphate is formed
(b) Zinc oxide is formed
(c) Zinc sulphide is formed
(d) No reaction takes place
Answer
A
Question. What is the another name of quick lime?
(a) Calcium hydroxide
(b) Calcium oxide
(c) Carbon dioxide
(d) Sodium oxide
Answer
B
Question. What is the chemical name for slaked lime?
(a) Calcium carbonate
(b) Calcium oxide
(c) Calcium hydroxide
(d) Carbon monoxide
Answer
C
Question. Why are articles made of iron painted to prevent rust?
(a) Paint makes articles made of iron beautiful
(b) Paint prevents iron articles to come in contact with moisture present in air.
(c) Paint prevents iron articles from getting sticky
(d) All of the above
Answer
B
Question. Which of the following is termed as rancidity?
(a) Reduction of oxygen present in food
(b) Oxidation of oil present in food
(c) Oxidation of sugar present in food
(d) All of the above
Answer
B
Question. How rancidity can be prevented?
(a) By adding antioxidants in food
(b) By adding more oxygen to food
(c) By keeping food items in open
(d) All of the above
Answer
A
Question. In which of the following category will you put the reaction of heating of calcium carbonate?
(a) Decomposition reaction
(b) Thermal decomposition reaction
(c) Endothermic reaction
(d) All of the above
Answer
D
Question. Which of the following product is formed after heating of limestone?
(a) Calcium oxide
(b) Calcium carbonate
(c) Hydrogen gas
(d) All of the above
Answer
A
Question. Balanced equation of Fe + H2O → Fe3O4 + H2 is
(a) Fe + 4H2O → Fe3O4 + H2
(b) Fe + 4H2O → Fe3O4 + 4H2
(c) 3Fe + H2O → Fe3O4 + 4H2
(d) 3Fe + 4H2O → Fe3O4+ 4H2
Answer
D
Question. The formula of quick lime and the compound formed when it reacts with water respectively are
(a) CaO, CaCO3
(b) CaO, Ca(OH)2
(c) Ca(OH)2, CaCO3
(d) CaCO3, Ca(OH)2
Answer
B
FILL IN THE BLANK
Question. Reactions in which heat is given out along with the products are called ………. reactions.
Answer
exothermic
Question. When calcium carbonate is heated, it decomposes to from ………. and ………. gas.
Answer
calcium oxide, carbon dioxide
Question. When an element displaces another element from its compound, a ………. reaction occurs.
Answer
displacement
Question. The addition of oxygen to a substance is called ……….
Answer
oxidation
Question. When calcium carbonate is heated, it decomposes to give ………. and ……….
Answer
CaO (s) and CO2 (g)
Question. Reactions in which energy is absorbed are known as ………. reactions.
Answer
endothermic
Question. The new substances produce in a reaction are called as ……….
Ans :
Answer
products
Question. In a ………. reaction two or more substances combine to form a new single substance.
Answer
combination
Case Study Based Questions
Read the following and answer any four questions from (i) to (v):
The reaction between MnO2 with HCl is depicted in the following diagram. It was observed that a gas with bleaching abilities was released.
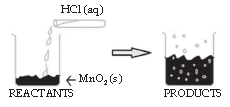
Question. The chemical reaction between MnO2 and HCl is an example of:
(a) displacement reaction
(b) combination reaction
(c) redox reaction
(d) decomposition reaction
Answer
C
Question. Chlorine gas reacts with _________ to form bleaching powder.
(a) dry Ca(OH)2
(b) dry CaO
(c) dil. solution of Ca(OH)2
(d) conc. solution of Ca(OH))2
Answer
A
Question. Identify the correct statement from the following:
(a) MnO2 is getting reduced whereas HCl is getting oxidized.
(b) MnO2 is getting oxidized whereas HCl is getting reduced.
(c) MnO2 and HCl both are getting reduced.
(d) MnO2 and HCl both are getting oxidized.
Answer
A
Question. In the above discussed reaction, what is the nature of MnO2?
(a) Acidic oxide
(b) Basic oxide
(c) Neutral oxide
(d) Amphoteric oxide
Answer
B

We hope the above multiple choice questions for Class 10 Science for Chapter 1 Chemical Reactions and Equations provided above with answers based on the latest syllabus and examination guidelines issued by CBSE, NCERT and KVS are really useful for you. Chemical Reactions and Equations is an important chapter in Class 10 as it provides very strong understanding about this topic. Students should go through the answers provided for the MCQs after they have themselves solved the questions. All MCQs have been provided with four options for the students to solve. These questions are really useful for benefit of class 10 students. Please go through these and let us know if you have any feedback in the comments section.
