Exam Question for Class 10 Science Chapter 2 Acids Bases Salts
Please refer to below Exam Question for Class 10 Science Chapter 1 Acids Bases Salts. These questions and answers have been prepared by expert Class 10 Science teachers based on the latest NCERT Book for Class 10 Science and examination guidelines issued by CBSE, NCERT, and KVS. We have provided Class 10 Science exam questions for all chapters in your textbooks. You will be able to easily learn problems and solutions which are expected to come in the upcoming class tests and exams for standard 10th.
Chapter 2 Acids Bases Salts Class 10 Science Exam Question
All questions and answers provided below for Exam Question Class 10 Science Chapter 2 Acids Bases Salts are very important and should be revised daily.
Exam Question Class 10 Science Chapter 2 Acids Bases Salts
Very Short Answer Type Questions
Question: ‘A’ is a soluble acidic oxide and ‘B’ is a soluble base. Compared to pH of pure water, what will be the pH of (a) solution of A (b) solution of B?
Answer: pH of pure water is 7. As A is a soluble acid, its pH will be less than that of pure water whereas pH of B will be more than that of pure water as it is a soluble base.
Question: An old person complained of acute pain in stomach. Doctor gave small antacid tablet and he got immediate relief. What actually happened?
Answer: As the old person was suffering from acidity, the antacid tablet, which is basic in nature, neutralized the excess acid in his stomach due to which he got immediate relief.
Question: A yellow powder X gives a pungent smell if left open in air. It is prepared by the reaction of dry compound Y with chlorine gas. It is used for disinfecting drinking water. Identify X and Y.
Answer: The yellow powder X is bleaching powder which is prepared by the reaction of chlorine on dry slaked lime [Ca(OH)2].
Question: Give reason: Cakes rise on adding baking powder
Answer: Baking powder is added to cakes to make them spongy. When baking powder is heated or mixed in water, the following reaction takes place:
NaHCO3 (aq) + H+ (from tartaric acid) →
CO2(g) + H2O(l) + Sodium salt of acid Carbon dioxide produced during the reaction
causes bread or cake to rise making them soft and spongy.
Question: Equal lengths of Mg ribbon are taken in test tubes A and B. Hydrochloric acid is added to test tube A. While acetic acid is added to test tube B. In which case the reaction would occur more vigorously and why?
Answer: The reaction will take place more vigorously in test tube A as hydrochloric acid is a stronger acid than acetic acid.
Question: Which bases are called alkalies? Give an example of alkalies.
Answer : Soluble bases are called alkalies, e.g. sodium hydroxide (NaOH) .
Question: Write a balanced chemical equation for a neutralisation reaction, mentioning the physical state of the reactants and the products.
Answer :

Question: The pH of a sample of vegetable soup was found to be 6.5. How is this soup likely to taste?
Answer : The taste will be slightly sour as it is weakly acidic.
Question: Write a balanced chemical equation for the reaction between sodium carbonate and hydrochloric acid indicating the physical state of the reactants and the products.
Answer :
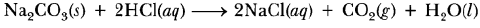
Question: What would be the colour of red litmus in a solution of sodium carbonate?
Answer : The red litmus will change to blue in sodium carbonate solution.
Question: What happens when chlorine is passed over slaked lime at 313K? Write chemical equation of the reaction involved and state two uses of the product obtained.
Answer : Bleaching powder is formed.
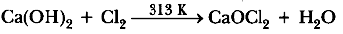
(i) It is used as bleaching agent in paper and textile industries.
(ii) It is used as disinfectant in purification of drinking water.
Question: Which gas is evolved when sodium hydrogencarbonate reacts with dilute hydrochloric acid?
Answer : Carbon dioxide gas is evolved.
Question: Name the gas usually liberated when a dilute acid reacts with a metal. What happens when a burning candle is brought near this gas?
Answer : H2 gas is liberated. It burns with pop sound when burning candle is brought near the gas.
Question: Curd is not kept in copper and brass utensils. Why?
Answer : Curd and sour substances contain acids which react with brass and copper vessels to form poisonous salts which are harmful for our health.
Question: What effect does an increase in concentration of H+(aq.) in a solution have on the pH of solution?
Answer : Higher the concentration, lower will be pH of the solution.
Question: Why does 1 M HC1 solution have a higher concentration of H+ ions than 1 M CH3COOH solution?
Answer : 1 M HCl has higher cone, of (H+) because it ionises completely in aqueous solution whereas CH3COOH does not as it is weak acid.
Question:. Which one of these has a higher concentration of H+ ions ? 1 M HCl or 1 M CH3COOH
Answer : 1 M HCl has higher concentration of H+ ions.
Question: What is the colour of litmus in a solution of ammonium hydroxide?
Answer : Red litmus will turn blue in ammonium hydroxide.
Question: Which gas is generally liberated when a dilute solution of hydrochloric acid reacts with an active metal?
Answer : Hydrogen gas is liberated when active metal reacts with dilute hydrochloric acid
Question: A student detected the pH of four unknown solution A, B, C and D as follows 11, 5, 7 and 2.
Predict the nature of the solution.
Answer. A is basic ‘B’ is acidic ‘C’ is natural and ‘D’ is strongly acidic.
Question: Name the natural source of each of the following acid
(i) Citric acid. (ii) Oxalic acid.
(iii) Lactic acid. (iv) Tartaric acid.
Answer : (i) Lemon and orange.(ii) Tomatoes and Guava.
(iii) Sour milk (curd) .(iv) Tamarind.
Question: How will you test for the gas which is liberated when hydrochloric acid reacts with an active \metal?
Answer. Bring a burning matchstick near the gas. It burns with ‘pop’ sound showing that it is hydrogen.
Question: (i) Give the constituents of baking powder
(ii) Why cake or bread swells on adding baking powder? Write chemical equation.
Answer. (i) Baking powder containg sodium hydrogen carbonate and tartaric acid.
(ii) It is due to carbon dioxide
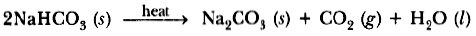
Question: Name the acid present in the following:
(i) Tomato (ii) Vinegar (iii) Tamarind
Answer. (i) Oxalic acid (ii) Acetic acid (iii) Tartaric acid
Question: (a) Define olfactory indicators. Name two substances which can be used as olfactory indicator.
(b) Choose strong acids from the following:
CH3COOH, H2SO4, H2CO3, HNO3
Answer. (a) Those substances whose smell (odour) changes in acidic or basic solution are called olfactory indicators, e.g. onion and vanilla.
(b) H2SO4 and HNO3 are strong acids.
Question: Explain how antacid works.
Answer. Hyperacidity is caused by excess of hydrochloric acid in stomach. Antacid is basic in nature. It neutralizes excess of acid and gives relief from pain caused by hyperacidity.
Question: State reason for the following statements:
(i) Tap water conducts electricity whereas distilled water does not.
(ii) Dry hydrogen chloride gas does not turn blue litmus red whereas dilute hydrochloric acid does.
(iii) During summer season, a milk man usually adds a very small amount of baking soda to fresh milk.
(iv) For a dilution of acid, acid is added into water and not water into acid.
(v) Ammonia is a base but does not contain hydroxyl group.
Answer. (i) Tap water contains ions which conduct electricity, distilled water does not contain ions.
(ii) Dry HCl does not form ions but HCl gives H+ and Cl–.
(iii) Baking soda does not allow milk to change to lactic acid which makes milk sour.
(iv) Adding water to acid is highly exothermic. Therefore water is added to acid very slowly with cooling.
(v) Ammonia dissolves in water and forms H– Therefore, it is basic in nature.
Question: A white coloured powder is used by doctors for supporting fractured bones.
(a) Write chemical name and formula of the powder.
(b) When this white powder is mixed with water a hard solid mass is obtained. Write balanced chemical equation for the change.
Answer.
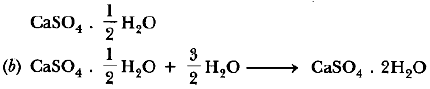
Question: Explain the action of dilute hydrochloric acid on the following with chemical equation:
(i) Magnesium ribbon (ii) Sodium hydroxide (iii) Crushed egg shells
Answer. (i) Hydrogen gas will be formed
Mg (g) + 2HCL(dil) → MgCI2 (aq) + H2(s)
(ii) sodium chloride and water will be formed
NaOH + HCI → NaCI + H2O
(iii) Crushed egg shell are made up of CaCO3 which reacts with dil HCl to give brisk effervescence due to CO2
CaCO3(s) + 2HCI → CaCI2 + H2O +CO2
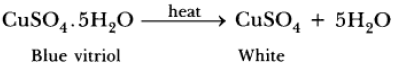
Question: (a) Write the chemical formula of hydrated copper sulphate and anhydrous copper sulphate. Giving an activity illustrate how these are inter convertible.
(b) Write chemical names and formula of plaster of paris and gypsum.
Answer. (a) CuSO4.5H2O is hydrated copper sulphate. CuSO4 is anhydrous copper sulphate.
Aim: To show crystalline salts contain water of crystallization.
Material Required: CuSO4.5H2O (Blue vitriol), boiling tube, burner, cork, delivery tube, test tube, clamp stand.
Procedure: 1.Take 2g of CuSO4.5H2O in a boiling tube fitted in a clamp stand.
2. Observe its colour. Fit it with cork and delivery tube bent at two right angles which dips into a test tube.
3. Heat crystals in boiling tube.
4. Observe vapours being condensed in test tube.
5. Cool the crystals and add few drops of water into it.
Observation: Water vapours get condensed in a test tube and colour of blue crystals changes into white. On adding water to anhydrous copper sulphate it changes into blue again.
Chemical Reaction :
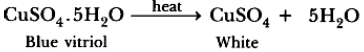
Conclusion : Crystalline substances have water of crystallization which are lost on heating. When we add water in CuSO4 till a saturated solution is formed. On cooling, it gets converted into CuSO4.5H2O crystals and it shows that both are inter convertible.

Short Answer Type Questions
Question: (a) State the chemical properties on which the following uses of baking soda are based:
(i) as an antacid
(ii) as a soda acid fire extiguisher
(iii) to make bread and cake soft and spongy.
Answer.
(b) How is washing soda is obtained from baking soda? Write balanced chemical equation.
(a) (i) It is weakly basic in nature and naturalize hyperacidity.
(ii) It liberates CO2 with H2SO4, which extinguish fire.
(iii) It liberates CO2 on heating which makes bread and cake soft and sponge.
Question: (a) Write the name given to bases that are highly soluble in water. Give an example.
(b) How is tooth decay related to pH? How can it be prevented?
(c) Why does bee sting cause pain and irritation? Rubbing of baking soda on the sting area gives relief. How?
Answer. (a) Alkali, e.g. NaOH (Sodium hydroxide).
(b) Lower the pH, more will be tooth decay. Acid reacts with Ca3(PO4)2 and cause tooth decay. It can be prevented by brushing teeth after every meal.
(ic) It is due to formic acid. Sodium hydrogencarbonate (Baking soda) neutralises formic acid giving relief.
Question: “Sodium hydrogencarbonate is a basic salt”. Justify the statement. How is it converted into washing soda? Explain.
Answer. Sodium hydrogen carbonate is a salt of sodium hydroxide (strong base) and carbonic
acid (weak acid). It is basic salt. It is converted into washing soda by heating followed by crystallization.

Question: Describe an activity with diagram to illustrate that the reaction of metal carbonates and metal bicarbonates with acids produces carbon dioxide. Write the relevant equations of all the reactions that take place. Name any two forms in which calcium carbonate is found in nature.
Answer. Aim: To show acid reacts with metal carbonate to liberate carbon dioxide,
Material Required: CaCO3 (marble chips), Woulfe-bottle, thistle funnel, dil. HCl, gas jar, matchbox, delivery tube bent at two right angles, lime water. Procedure:
Take two test tubes, label them as A and B.
Take about 0.5 g of sodium carbonate (Na2CO3) in test tube A and about 0.5 g of sodium hydrogen carbonate (NaHCO3) in test tube B.
Add about 2 mL of dilute HCl to both the test tubes.
Pass the gas produced in each case through lime water (calcium hydroxide solution) as shown in below figure and record your observations.

Conclusion: Metal carbonates react with dilute acids to liberate carbon dioxide. Limestone, chalk, marble are different forms of calcium carbonate. All metal carbonates and hydrogen carbonates react with acids to form corresponding salts, water and carbon dioxide.
Question: (i) Explain why is hydrochloric acid a strong acid and acetic acid, a weak acid. How can it be verified?
(ii) Explain why aqueous solution of an acid conducts electricity.
(iii) You have four solutions A, B, C and D. The pH of solution A is 6, B is 9, C is 12 and D is 7,
(a) Identify the most acidic and most basic solutions.
(b) Arrange the above four solutions in the increasing order of H+ ion concentration.
(c) State the change in colour of pH paper on dipping in solution C and D.
Answer. (i) HCl is completely ionised in aqueous solution whereas acetic acid is partially ionised in aqueous solution. HCl gives dark red colour with pH paper whereasCH3COOH gives orange colour
(ii) It is because acid ionises in aqueous solution and these ions conduct electricity. (Hi) (a) ‘A’ is most acidic and ‘C’ is most basic.
(b) C (10-12) < B (10-9) < D (10-7) < A (10-6)
(c) pH paper will become blue in ‘C’ and green in ‘D’.
Question: (a) Identify the compound of calcium which is yellowish white powder and is used for disinfecting drinking water. Write its chemical name and formula. How is it manufactured?
Write the chemical equation for the reaction involved. Also list two other uses of the compound.
(b) Write the balanced chemical equation qf chlor-alkali process.
Answer. (a) The compound is bleaching powder (CaOCl2). Its chemical name is calcium oxychloride. It is manufactured by reaction of solid slaked lime with dry chlorine gas. give relief from acidity. Write the name of one such antacid.
(b) Fresh milk has a pH of 6. How does the pH will change as it turns to curd? Explain your answer.
(c) A milkman adds a very small amount of baking soda to fresh milk. Why does this milk take a longer time to set as curd?

Question: (a) Identify the acid and the base whose combination forms the common salt that you use in your food. Write its formula and chemical name of this salt. Name the source from where it is obtained.
(b) What is rock salt? Mention its colour and the reason due to which it has this colour.
(c) What happens when electricity is passed through brine? Write the chemical equation for it.
Answer. (a) HCl is acid and NaOH is base whose combination forms the common salt. Its formula is NaCl
(Sodium chloride). It is obtained from sea water.
(b) Rock salt is the common name for the mineral “halite”. Its chemical formula is NaCl.
It may be white or light blue or yellow depending upon impurities present in it.
Question: (a) Crystals of a substance changed their colour on heating in a closed test tube but regained it after sometime when they were allowed to cool down. Name the substance and write its formula and explain the phenomenon involved.
(b) Name the compound whose one formula unit is associated with 10 water molecules. How is it prepared? Give equations of related reactions. Give two uses of the compound.
Answer. (a) CuSO4.5H2O is a blue crystalline solid. It becomes dirty white on heating due to loss of water molecules and it becomes amorphous. It regains its colour by absorbing water from atmosphere
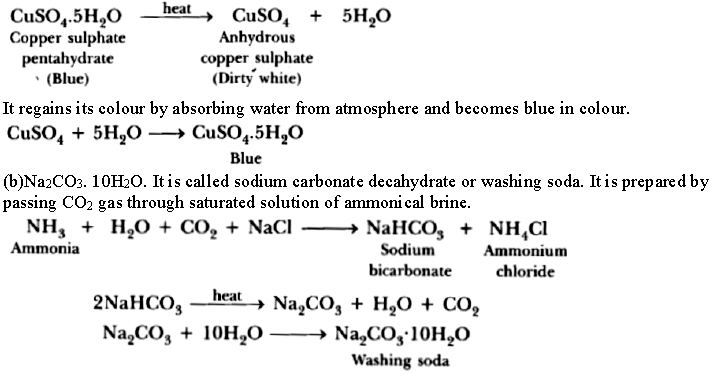
Uses:
(i) It is used in the production of washing powder.
(ii) It is used for the manufacture of glass.
Question: (a) Mention the pH range within which our body works. Explain how antacids give relief from acidity. Write the name of one such antacid.
(b) Fresh milk has a pH of 6. How does the pH will change as it turns to curd? Explain your answer.
(c) A milkman adds a very small amount of baking soda to fresh milk. Why does this milk take a longer time to set as curd?
(d) Mention the nature of toothpastes. How do they prevent tooth decay?
Answer. (a) Our stomach has pH equal to 2. Antacids neutralizes excess of acid in our body and gives relief from hyperacidity. Sodium hydrogencarbonate is one of such antacid.
(b) pH will decrease as it turns to curd because curd is acidic due to the presence of lactic acid.
(c) It takes longer time to set as curd as bacteria do not work well in presence of sodium hydrogencarbonate, i.e. fermentation will take place slowly.
(d) Toothpastes are basic in nature. They neutralize the acid formed in mouth which causes tooth decay.
Question: a) A metal compound ‘X’ reacts with dil. H2SO4 to produce effervescence, The gas evolved extinguishes a burning candle. If one of the compound formed is calcium sulphate, then what is ‘X’ and the gas evolved? Also, write a balanced chemical equation for the reaction which occurred.
(b) (i) Name one antacid. How does it help tq relieve indigestion in stomach?
(ii) A farmer treats the soil with quicklime or calcium carbonate. What is the nature of soil?
Why does the farmer treat the soil with quicklime?
Answer. (a) ‘X’ is CaCO3 (calcium carbonet) the gas evolved is CO2
CaCO3 + H2SO4(dil.) —> CaSO4 + H2O + CO2
calcium sulphase
(b) (i) NaHCO3 is antacid. It neutralizes excess of acid formed in the stomach.
(ii) The soil is acidic in nature. The farmer wants to make it neutral by adding quicklime which is good for crops.
Question: What are strong and weak acids? In the following list of acids, separate strong acids from weak acids. Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
Answer. Strong acids are those acids which are completely ionised in aqueous solution. Weak acids are those which do not ionise completely in aqueous solution. Strong acid: HCl,
HNO3,H2SO4 Weak acid: Citric acid, acetic acid, formic acid.
Question: (a) Explain the following with the help of balanced chemical equations only.
(i) When an acid reacts with a metal carbonate.
(ii) When an acid reacts with a metal bicarbonate.
(iii) When an acid reacts with a metal oxide.
(b) You are given three solutions A, B and C with pH values 2, 10 and 13 respectively. Write which solution has more hydrogen ion concentration among the three and state the nature ‘acidic or basic’ of each solution.
Answer.

Question: State in brief the preparation of washing soda from baking soda. Write balanced
chemical equation of the reaction involved.
Answer. Sodium hydrogencarbonate (baking soda) on heating gives sodium carbonate which on recrystallisation gives washing soda.

Baking soda on heating gives sodium carbonate which on crystallisation from aqueous solution gives washing soda, e.g.
Na2CO3 + 10H2O → Na2CO3 . 10H2O
Question: State the chemical name of Plaster of Paris. Write a chemical equation to show the reaction between Plaster of Paris and water.
Answer. Calcium sulphate hemihydrate.
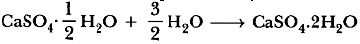
Question: State reasons for the following statements:
(i) Stain of curry on a white cloth becomes reddish brown when soap is scrubbed on it and turns yellow again when the cloth in washed with plenty of water.
(ii) Curd should not be kept in copper or brass vessels. What is done to protect it?
Answer. (i) Turmeric reacts with sodium hydroxide present in soap to form red coloured compound. It turns yellow again because sodium hydroxide becomes very dilute on adding lot of water and reaction stops.
(ii) Curd contains lactic acid which reacts with copper or brass vessels and taste changes. Curd should be kept in glass, steel or ceramic container which does not react with lactic acid present in it.
Question: Classify the following salts into acidic, basic and neutral: Potassium sulphate, ammonium
chloride, sodium carbonate, sodium chloride.
Answer. Neutral: Potassium sulphate, Sodium chloride Acidic: Ammonium chloride Basic:
Sodium carbonate
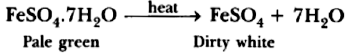
Question: What is the colour of FeSO4.7H2O crystals? How does this colour change upon heating?
Give balanced chemical equation for the changes.
Answer. Pale green is the colour of FeSO4.7H2O crystals. It becomes dirty white on heating.
Question: A student dropped few pieces of marble in dilute HC1 contained in a test tube. The evolved gas was passed through lime water.
(i) What change would be observed in lime water?
(ii) Write balanced chemical equation for the above change.
Answer. (i) Lime water will turn milky due to formation of calcium carbonate.
(ii) Ca(OH)2 (aq) + CO2 (g) —> Ca CO3 (s) + HaO(l)
Question: A gas ‘X’ reacts with lime water and forms a compound ‘Y’ which is used as a bleaching agent in chemical industry. Identify ‘X’ and ‘Y\ Give the chemical equation of the reactions involved.
Answer. ‘X’ is chlorine; ‘Y’ is bleaching powder.

Question: (a) What is universal indicator?
(b) Write the chemical equation involved in the preparation of sodium hydroxide. Name the process.
Answer. (a) Universal indicator is the mixture of synthetic indicators which is used to find pH of solutions.

Question: What is a neutralisation reaction? Give two examples.
Answer. The reaction between acid and base to form salt and water is called neutralization reaction. e.g. NaOH + HCl → NaCl + H2O and 2NaOH + H2SO4 → Na2SO4 + 2H2O
Question: (i) Name the compound which is obtained from baking soda and is used to remove permanent hardness of water.
(ii) Write its chemical formula.
(iii) What happens when it is recrystallised from its aqueous solution?
Answer. (i) Sodium carbonate is obtained from baking soda and is used to remove hardness of water.
(ii) Na2CO3 .
(iii) It changes to washing soda,Na2CO3. 10H2O .
Question: What is Plaster of Paris chemically? How is it prepared? List its two important uses.
Answer. Calcium sulphate hemihydrate.
It is prepared by heating gypsum at 373 K.

(i) It is used to prepare chalks.
(ii) It is used to make casts and moulds.
Question: Name the products formed in each case when
(a) hydrochloric acid reacts with caustic soda.
(b) granulated zinc reacts with caustic soda.
(c) carbon dioxide is passed into lime water.
Answer.

A white powder is added while baking breads and cakes to make them soft and fluffy. Write the name of the powder. Name its main ingredients. Explain the function of each ingredient.
Write the chemical reaction taking place when the powder is heated during baking.
Answer. Baking powder.
It consist of sodium hydrogen carbonate and tartaric acid.
Sodium hydrogen carbonate gives CO2 which makes cake soft and fluffy. Tartaric acid neutralizes the bitterness due to sodium carbonate produced.
Question: What is tooth enamel chemically? State the condition when it starts corroding. What happens when food particles left in the mouth after eating degrade? Why do doctors suggest use of tooth powder/toothpaste to prevent tooth decay?
Answer. It is made up of calcium phosphate.
It starts corroding due to acid formed in mouth. The food particles which are left in mouth form acids which cause tooth decay. Toothpaste and tooth powder are basic and neutralise acid formed in mouth which prevents tooth decay.
Question: Compounds like alcohols and glucose also contain hydrogen but are not categorised as acids. Discuss an activity to prove it.
Answer. Take a beaker of 250 ml and place two nails fixed with the help of cork.
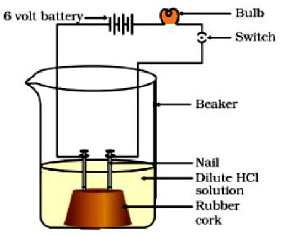
• Connect the nails to the two terminals of a 6 volt battery as shown in figure.
• Now add some water containing ethanol and put the switch ON.
• Repeat the experiment with glucose solution.
Observation : K The bulb will not glow and the needle of ammeter will not show deflection because glucose and ethanol do not conduct electricity.
Conclusion: The experiment shows glucose and ethanol do not ionise in aqueous solution, that is, they do not give H+ ions, therefore cannot conduct electricity. Thus, glucose and ethanol are not categorised as acids.
Question: What is meant by ‘water of crystallisation’ of a substance ? Describe an activity to show that blue copper sulphate crystals contain water of crystallisation.
Answer. The water molecules associated with a crystalline substance is called ‘water of crystallisation’.
To show crystalline salts contain water of crystallisation.
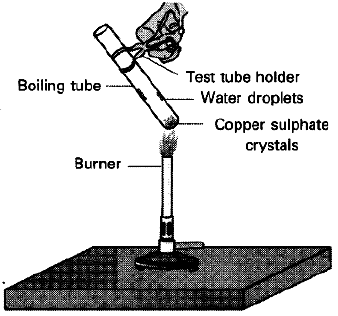
Materials Required: CuS04.5H20 (Blue vitriol), boiling tube, burner, cork, delivery tube, test tube, clamp stand.
Procedure:
1. Take 2g of CuS04.5H20 in a boiling tube fitted in a clamp stand.
2. Observe its colour. Fit it with cork and delivery tube bent at two right angles which dips into a test tube.
3. Heat crystals in boiling tube. ‘
4. Observe vapours being condensed in test tube.
5. Cool the crystals and add few drops of water into it.
Observation: Water vapours get condensed in a test tube and colour of blue crystals changes into white. On adding water to anhydrous copper sulphate, it changes into blue again.
Chemical Reaction:
Blue vitriol White
Conclusion: Crystalline substances have water of crystallisation which are lost on heating.
Question: What is baking soda chemically called? Give reaction involved in its preparation. Write one of its uses.
Answer. Sodium hydrogen carbonate.

It is used as an antacid.
Question: (a) What is an alkali? Give an example.
(b) Why do HCl, HNO3, etc. show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Answer. (a) Soluble bases arp called alkalies, e.g. sodibm hydroxide is an alkali.
(b) HCl, HNO3ionise in aqueous solution, whereas alcohol and glucose do not show acidic characters because they do not ionise in aqueous solution.

Question: Give reasons for the following:
(A) Only one half of water molecule is shown in the formula of Plaster of Paris.
(B) Sodium hydrogen carbonates is used as an antacid.
(C) On strong heating, blue coloured copper sulphate crystals turn white.
Answer: (A) Only one half of water molecule is shown in the formula of plaster of Paris because two formula units of CaSO4 share one molecule of water.
Explanation: Formula CaSO4.1/2 H2O
Chemical name calcium sulphate hemihydrate
Common name Plaster of Paris (POP).
When gypsum is heated at 373 K, it loses 1 1/2
molecules of water and become CaSO4 . 1/2 H2O.
(B) Sodium hydrogen carbonate is used as an antacid because it is alkaline in nature and neutralises excess acid in stomach and provides relief.
(C) Blue coloured copper sulphate crystals on strong heating loses 5 molecules of water of crystallisation and changes to anhydrous copper sulphate which is white in colour.
CuSO4.5H2O(s) Hea→t CuSO4(s) + 5H2O(l)
Pentahydrate copper Anhydrous copper
sulphate sulphat
(Blue) (White)
Explanation: Blue copper sulphate crystals is a hydrated salt which on heating changes to white anhydrous copper sulphate and 5 molecules of water of crystallisation appear on the upper cooler parts inside the test tube. If we put 2-3 drops of water on white crystals, they again turn blue.
Question: Match the acids given in column I with their correct source given in column II:

Answer:

Explanation: Lactic acid sources: Lactic acid is found in products such as cheese, yogurt, soy sauce and pickled vegetables.
Acetic acid sources: Acetic acid is found in condiments such as ketchup, mayonnaise and mustard.
Citric acid sources: Citric acid is found in citrus fruits, tomatoes and pineapples.
Oxalic acid sources: Oxalic acid is found in leafy green vegetables and beets.
Question: (A) Dry pellets of a base ‘X’ when kept in open absorbs moisture and turns sticky. The compound is also formed by chloralkali process. Write the chemical name and formula of X. Describe chloralkali process with balanced chemical equation. Name the type of reaction that occurs when X is treated with dilute hydrochloric acid.Write the chemical equation.
(B) While diluting an acid, why is it recommended that the acid should be added to water and not water to acid?
Answer: (A) The common base which absorbs moisture and becomes sticky and is also a by-product of chloralkali process is sodium hydroxide (NaOH).
When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor- alkali process because of the products formed: chlor for chlorine and alkali for sodium hydroxide. Following is the chemical equation for the process 2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
When NaOH is treated with HCl, NaCl is formed.
NaOH + HCl → NaCl + H2O
It is a type of neutralization reaction.
(B) Dissolution of acid into water is an exothermic process, hence when we dissolve an acid into water we prefer that acid is added to water as it will be less exothermic and production of large heat and splashing of acid will be ignored, as it can happen when we add water to acid.
Question: A student prepared solutions of (i) an acid and (ii) a base in two separate beakers. She forgot to label the solutions and litmus paper is not available in the laboratory. Since, both the solutions are colourless, how will she distinguish between the two?
Answer: In the absence of litmus, other natural or synthetic substances can be used to test acid and bases. Such substances are called indicators. Indicators such as methyl orange and phenolphthalein can be used to test the nature of a solution. These indicators show change in their colour in acidic, neutral and basic solutions. We can also use natural indicators such as turmeric and grape juice. A few indicators with characteristic colour change are shown below:

Question: Fill in the missing data in the given table.

Answer:

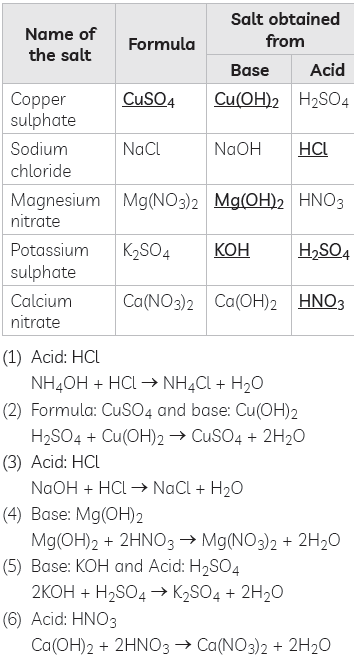
Question: Give the chemical name of the compound present in tooth enamel. What is the nature of this compound?
Answer: The tooth enamel is made of calcium phosphate [Ca3(PO4)2]. It is basic in nature. It starts getting corroded when the pH in the mouth is slightly acidic. This is the reason why toothpaste contain bases to neutralise the acid in the mouth.
Question: Hydrochloric acid reacts with a metal X to form a gas Y, which burns with a ‘pop’ sound.
Sodium hydroxid solution also reacts with same metal X to form same gas Y.
(A) Name X and Y.
(B) Write the chemical equation of the reaction of metal X with (i) HCl and (ii) NaOH solution.
Answer: When an acid reacts with a metal, then salt and hydrogen gas are formed.
Metal + Acid → Salt + H2 gas
Zn + 2HCl → ZnCl2 + H2↑
Similarly, when sodium hydroxide reacts with zinc metal, hydrogen gas is formed.
2NaOH + Zn Heat→ Na2ZnO2 + H2↑
Sodium Zinc Sodium Hydrogen
hydroxide zincate (gas)
(base) (salt)
Thus, from the above equations, it is clear that X is zinc metal and Y is hydrogen gas.
Question: A metal compound X reacts with dilute hydrochloric acid to produce brisk efferve- scence. The gas evolved forms a white precipitate when passed through lime water. Write balanced chemical equations involved in the above mentioned chemical reactions.
Answer: • MCO3 + 2HCl → MCl2 + H2O + CO2↑
(X) Brisk Effervescence
• Ca(OH)2 + CO2 → CaCO3↓ + H2O
White ppt
Question: Sabina studied in history about Mahatma Gandhi’s Dandi March and came to know that sodium chionde was an important symbol in our struggle for freedom she started exploring from the internet as she was anxious to know what all we can do with this salt other than using it as a table salt to make food tasty.
Table salt is also used to preserve food, facilitates transport of nutrients and oxygen and used as raw material to form various salts. Based on the understanding of the given passagte and the related studied concepts, answer the following questions:
(A) Name two salts of daily use for which common salt (sodium chloride) is the raw material?
(B) Draw a diagram of chloralkali process and write its equation.
Answer: (A) Two salts of daily use which can be prepared from common salt are: baking soda and washing soda.
(B)

Explanation: When electricity is passed through an aqueous solution of sodiuchloride (called brine), it decomposes to form sodium hydrogen. The products formed are alkali (sodium hydroxide) chlorine (chlor) and the process was named as chlor-alkali process.
Question: (A) Under what condition does a farmer need to treat the soil on his field with quick lime or slaked lime or chalk?
(B) You are given two solutions A and B and their pH is 6 and 8 respectively.
Answer the following:
(i) Which of the two solutions have more hydrogen ion concentration?
(ii) Which is acidic and which is basic?
(C) What is dilution?
Answer: (A) If the soil that is on the field is too acidic then the farmer should treat the soil with quicklime or slaked lime or chalk as they are ll alkaline substances and adding them to the soil will neutralize the acidic nature of soil.
Explanation: For healthy growth of plants,the soil should be neither alkaline nor highly acidic.
(B) pH of solution A = 6
pH of solution B = 8
(i) Solution A will have the higher hydrogen ion concentration.
Explanation: We know that the pH of anysolution is inversely proportional to the hydrogen ion concentration. This means that the solution that has lower pH number will have the higher hydrogen ion concentration.
(ii) Also Solution A is acidic and solution B is basic because H+ ion concentration is higher in acidic solutions.
(C) Mixing an acid or base with water results in decrease in concentration of ions (H3O+/ OH–) per unit volume. Such process is called dilution and the acid or base is said to be dituted. The process of dissolving an acid or a base in water is a highly exothermic reaction.
Long Answer Type Question:
Question: A dry pellet of a common base B when kept in open absorbs moisture and turns sticky. The compound is also a product of chlor-alkali process. Identify B. What type of reaction occurs when B is treated with an acidic oxide?
Write a balanced chemical equation for one such solution.
Answer: Base B is sodium hydroxide (NaOH). Dry pellets of sodium hydroxide (NaOH) absorb moisture from the atmosphere and become sticky. It is also a by-product of chlor-alkali process.
Chlor-alkali process is the electrolysis of brine (saturated solution of sodium hydroxide) that forms aqueous sodium hydroxide with hydrogen gas and chlorine gas.
The acidic oxide such as CO2 reacts with a base such as NaOH to give salt (sodium carbonate) and water. Such a reaction is called neutralisation reaction.
2NaOH + CO2 → Na2CO3 + H2O
Sodium Sodium
hydroxide carbonate
(B)
Thus, B is NaOH and reaction between B and acidic oxide is called a neutralization reaction.
Question: State the effect of concentration of H+(aq) ions on the nature of the solution. Do basic solutions also have H+(aq) ions ? If yes, then why are these basic?
Answer: The solution becomes more acidic when the concentration of H+ (aq) ions increases.
Yes, basic solutions also have H+ (aq) ions.
They are basic as the concentration of OH– ions is more than that of H+ ions in the solution.
Question: (A) What does pH scale measure?
(B) Write its range.
(C) State the significance of highest and lowest values of pH scale.
OR
(A) Why is electrolysis of brine called ‘Chlor- alkali process’? Write the chemical equation involved in this process.
(B) A few crystals of hydrated copper sulphate are heated in a dry test-tube. Enlist any two observations.
Answer: (A) pH scale measures the hydrogen ion con-centration in a solution thus indicating acidic/basic nature of a solution.
(B) From 0 to 14
(C) Significance:
Highest value – very basic/alkaline solution.
Lowest value – very acidic solution.
OR
(A) The products formed are ‘chlor’ for chlorine and ‘alkali’ for sodium hydroxide.
2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
(B) Two observations:
(i) Water droplets in the boiling tube.
(ii) Change in colour from blue to white.
Question: Define water of crystallisation. Give the chemical formula for two compounds as examples. How can it be proved that the water of crystallisation makes a difference in the state and colour of the compounds?
Answer: Water of crystallisation: It is the fixed number of water molecules present in one formula unit of a salt.
For Examples: copper sulphate crystals CuSO4.5H2O – copper sulphate crystals contain 5 molecules of water of crystallisation in one formula unit.
Sodium carbonate crystals (washing soda crystals) contain 10 molecules of water and its formula is Na2CO3.10H2O.
The water of crystallisation gives the crystals of the salts. Their shape and colour. For example,the presence of water of crystallisation in copper sulphate crystals imparts them blue colour and crystalline shape.
Heat a few crystals of CuSO4.5H2O which are blue in colour in a dry boiling tube.
On heating, the blue copper sulphate crystals turn white and a powdery substance is formed.
Tiny droplets of water are seen in the boiling tube.
CuSO4.5H2O → CuSO4 + 45H2O
Blue in colour, copper sulphate crystals powdery substance White in colour, powdery substance Cool the boiling tube add 2-3 drops of water on the white copper sulphate powder. The crystals of copper sulphate become blue in colour.
CuSO4 + 45H2O → CuSO4.5H2O

Question: Blue litmus solution is added to two test tubes A and B containing dilute HCl and NaOH solution respectively. In which test tube a colour change will observed? State the colour change and give its reason.
Answer: Colour change will be observed in Test tube A containing dil HCl.
It will change the colour of blue litmus to red.
Test tube contains dil HCl which is an acid and acids turn the colour of blue litmus to red as they contain H+ ions.
Question: 1 g of solid sodium chloride is taken in a clean and dry test tube and 2 mL of conc. sulphuric acid is added to it. If the gas evolved is tested first with dry and then with wet blue litmus paper, in which case will the litmus paper change colour? Give reason for your answer.
What inference can be drawn about the nature of the evolved gas? Support your answer with chemical equation for the reaction.
Answer: The gas evolved by the reaction between sodium chloride and conc. Sulphuric acid is HCl gas.
Chemical equation for the reaction taking place is:
2NaCl + H2SO4 → Na2SO4 + 2HCl
When we test the HCl gas evolved, only the wet blue litmus paper will change colour to red.
The hydrogen ions are produced by HCl gas only in the presence of water. The reaction
taking place is:
HCl + H2O → H3O+ + Cl–
The inference drawn is that HCl gas is acidic in nature.
Question: Answer the following:
(A) State the relation between hydrogen ion concentration of an aqueous solution and its pH.
(B) An aqueous solution has a pH value of 7.0. Is this solution acidic, basic and neutral?
(C) Which has a higher pH value, 1 M HCl or 1 M NaOH solution?
(D) Tooth enamel is one of the hardest substances in our body. How does it undergo damage due to eating chocolates and sweets? What should we do to prevent it?
(E) How do [H+] ions exist in nature?
Answer: (A) Relation between hydrogen ion concentration of an aqueous solution and its pH: If H+ ion concentration is more, pH will be less because pH of a solution is inversely proportional to H+ ions concentration.
If [H+] = 10–x M,
then pH = x
(B) An aqueous solution has a pH value of 7.0. The solution is neutral.
(C) 1 M HCl means
[H+] = 100 M
Therefore pH = 0
1 M NaOH means
[OH–] = 1 M
Therefore [H+] =10−14 /[OH− ]= 10–14 M
pH = 14
Hence, 1 M NaOH solution has more pH value.
(D) Tooth enamel is made up of calcium phosphate which gets corroded when the pH in the mouth is below 5.5. Bacteria present in the mouth produce acids by degradation of sugar and food particles in the mouth after eating. It can be prevented by using Tooth pastes, which are basic.
(E) H+ ions do not exist independently as it gains the unshared electron pairs on the oxygen in the water molecule to form a hydronium ion.
H+ + H2O → H3O+
Question: (A) Draw a neat and labeled diagram to show the action of dilute sulphuric acid on zinc granules.
(B) Write a chemical equation when dilute sulphuric acid reacts with zinc granules.
State the type of the reaction.
(C) Name the gas evolved. How will you test for the gas?
Answer: (A) Action of dilute sulphuric acid on zinc granules.

(B) H2SO4 + Zn → ZnSO4 + H2(g)
The type of reaction is displacement reaction metal in displaces hydrogen from the acid (H2SO4) as hydrogen gas (H2) and forms a compound (salt).
(C) The gas evolved is hydrogen.
We can test for the hydrogen gas by passing it through soap solution and then bring a burning candle near the soap bubbles filled with gas. Hydrogen gas will burn with pop sound.
Question: What is observed when 2 mL dilute hydrochloric acid is added to 1 g of sodium carbonate taken in a clean and dry test tube?
Write chemical equation for the reaction involved.
Answer: When 2 mL of dil. HCl is added to 1 g of Na2CO3 in a test tube, we will observe a brisk effervescence as carbon dioxide gas is produced, along with salt and water.
The chemical equation for the reaction involved is :
Na2CO3 (s) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
Question: For making cake, baking powder is taken. If at home your mother uses baking soda instead of baking powder in cake.
(A) How will it affect the taste of the cake and why?
(B) How can baking soda be converted into baking powder?
(C) What is the role of tartaric acid added to baking soda?
Answer: (A) Baking soda is sodium hydrogen carbonate (NaHCO3). It decomposes to sodium carbonate, water and carbon dioxide on heating.
2NaHCO3 → Na2CO3 + H2O + CO2
Baking powder is a mixture of sodium hydrogen carbonate (NaHCO3) with tartaric acid. It readily reacts with sodium carbonate and neutralizes it.
Therefore use of baking soda will give a bitter taste to cake due to the presence of sodium carbonate as sodium carbonate is basic in nature.
(B) Baking powder is formed by addition of tartaric acid to baking soda.
(C) Presence of tartaric acid in baking powder neutralizes the effect of sodium carbonate formed during decomposition of baking soda. Tartaric acid is added to neutralize the bitterness produced by the baking powder.
Also when baking powder mixes with water, then the sodium hydrogen carbonate reacts with tartaric acid to evolve carbon dioxide gas which gets trapped in the wet dough and bubbles out slowly making the cake to rise and hence ‘soft and spongy’ thus endowing them with a light, fluffy texture.
The equation which takes place can be shown as:
NaHCO3 + H+ → Na+ + CO2 + H2O

