Exam Question for Class 12 Chemistry Chapter 1 The Solid State
Please refer to below Exam Question for Class 12 Chemistry Chapter 1 The Solid State. These questions and answers have been prepared by expert Class 12 Chemistry teachers based on the latest NCERT Book for Class 12 Chemistry and examination guidelines issued by CBSE, NCERT, and KVS. We have provided Class 12 Chemistry exam questions for all chapters in your textbooks. You will be able to easily learn problems and solutions which are expected to come in the upcoming class tests and exams for standard 12th.
Chapter 1 The Solid State Class 12 Chemistry Exam Question
All questions and answers provided below for Exam Question Class 12 Chemistry Chapter 1 The Solid State are very important and should be revised daily.
QUESTION BANK
Question.Which type of defect has the presence of cations in the interstitials sites?
(a) Schottky defect
(b) Vacancy defect
(c) Frenkel defect
(d) Metal deficiency defect
Answer
C
Question.Which one of the following is a molecular crystal?
(a) Rock salt
(b) Quartz
(c) Dry ice
(d) Diamond
Answer
C
Question.Which of the following metal oxides is antiferromagnetic in nature?
(a) MnO2
(b) TiO2
(c) VO2
(d) CrO2
Answer
A
Question.The crystal with metal deficiency defect is
(a) NaCl
(b) FeO
(c) KCl
(d) ZnO
Answer
B
Question.Which of the following compound is metallic and ferromagnetic?
(a) CrO2
(b) VO2
(c) MnO2
(d) TiO2
Answer
A
SECTION –B
Question.Why is Frenkel defect not found in pure alkali metal halides?
Answer.Frenkel defect is not found in alkali metal halides because the ions cannot get into the interstitials sites due to their larger size.
Question.Why does the electrical conductivity of semiconductor increases with rise in temperature?
Answer.In case of semiconductors, the conductivity is due to the presence of impurity and defects. As the number of defects increases with rise in temperature, the conductivity increases.
Question.Analysis shows that nickel oxide has the formula Ni.98O1.00. What fractions of the Nickel exist as Ni+2and Ni+3?
Answer.8.Let Ni+2 be x so that Ni+3will be .98-x. Total charge on the compound must be zero so that
2x+3(.98-x)-2=0
2x+2.94-3x-2=
X=0.94

%of Ni+3=4%
Question.Why does ZnO appear golden yellow at high temperature? Explain?
Answer.When ZnO is heated it loses oxygen as : ZnO →Zn+2+2e–.The Zn+2 ions are entrapped in the interstitial sites and electrons are entrapped in the neighbouring interstitial sites to maintain electrical neutrality. This results in metal excess defect. Due to the presence of electrons in the interstitial void, the colour is yellow.
Question.Calculate the packing efficiency in face centered cubic arrangement?
Answer.Suppose the edge length of the unit cell=a
Radius of each sphere=r In case of fcc a= 2√2r
Volume of unit cell = a3 = (2√2r)3 = 16√2r3

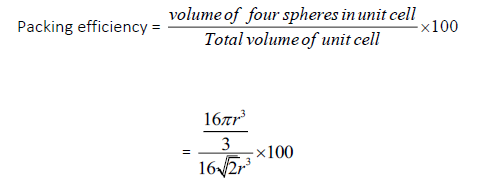
Packing efficiency =74%
SECTION –C
Question.Why does LiCl acquire pink colour when heated in Li vapours ?
Answer.On heating LiCl in Li vapours the excess of Li atoms deposit on the surface of crystal.The Cl-ions diffuses to the surface of the crystal and combine with Li atoms to form LiCl.The electrons produced by ionization of Li atoms diffuse into the crystal and get trapped at anion vacancies called F centres .These absorb energy from visible light and radiate pink colour.
Question.Calculate the number of unit cells in 8.1 g of aluminium if it crystallizes in a face centered Cubic (f.c.c) structure.(Atomic mass of Al =27 g mol–)
Answer.
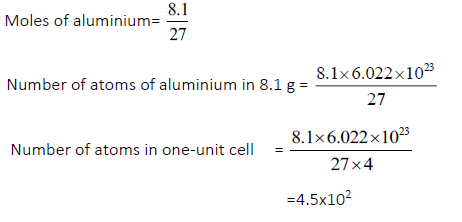
Question.An element having bcc geometry has atomic mass 50u.Calculate the density of the unit cell ,if its edge length is 290 pm.
Answer.13.Length of edge a=290 pm=290×10-10 cm
Since it is a bcc arrangement
Number of atoms in the unit cell=2
Atomic mass of the element M=50 gmol–
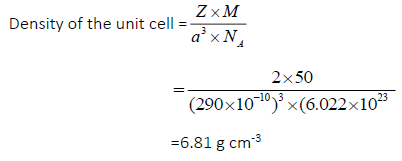
Question.An element with molar mass 27g mol-forms a cubic unit cell with edge length 4.05×10-8cm.If its density is 2.7 g cm-3,what is the nature of unit cell ?
Answer.

Since Z=4 It is a fcc arrangement
Question. An element crystallizes in a f.c.c. lattice with cell edge of 400 pm. The density of the unit cell is 7 g cm-3.How many atoms are present in 280 g of the element?
Answer.Edge length a=400 pm =400×10-10cm
Volume of unit cell =(400×10-10cm)3=64×10– 24cm3

For fcc No of atoms per unit cell=4
Number of atoms present in 280 g of element=6.25×1023x4=2.5×1024 atoms.
