Exam Question for Class 12 Chemistry Chapter 2 Solutions
Please refer to below Exam Question for Class 12 Chemistry Chapter 2 Solutions. These questions and answers have been prepared by expert Class 12 Chemistry teachers based on the latest NCERT Book for Class 12 Chemistry and examination guidelines issued by CBSE, NCERT, and KVS. We have provided Class 12 Chemistry exam questions for all chapters in your textbooks. You will be able to easily learn problems and solutions which are expected to come in the upcoming class tests and exams for standard 12th.
Chapter 2 Solutions Class 12 Chemistry Exam Question
All questions and answers provided below for Exam Question Class 12 Chemistry Chapter 2 Solutions are very important and should be revised daily.
OBJECTIVE TYPE MCQs
Question. Which of the following statements is incorrect?
(a) A solution in which no more solute can be dissolved at the same temperature and pressure is called a saturated solution.
(b) An unsaturated solution is one in which more solute can be dissolved at the same temperature.
(c) The solution which is in dynamic equilibrium with undissolved solute is the saturated solution.
(d) The minimum amount of solute dissolved in a given amount of solvent is its solubility
Answer
D
Question. The relative lowering of the vapour pressure is equal to the ratio between the number of
(a) solute molecules to the solvent molecules
(b) solute molecules to the total molecules in the solution
(c) solvent molecules to the total molecules in the solution
(d) solvent molecules to the total number of ions of the solute.
Answer
B
Question. Which is an application of Henry’s law?
(a) Spray paint
(b) Bottled water
(c) Filling up a tyre
(d) Soft drinks (soda)
Answer
D
Question. The molarity of the solution containing 7.1 g of Na2SO4 in 100 ml of aqueous solution is
(a) 2 M
(b) 0.5 M
(c) 1 M
(d) 0.05 M
Answer
B
Question. Which of the following liquid pairs shows a positive deviation from Raoult’s law?
(a) Water – Nitric acid
(b) Benzene – Methanol
(c) Water – Hydrochloric acid
(d) Acetone – Chloroform
Answer
B
ASSERTION-REASON TYPE QUESTIONS
Directions: Each of these questions contain two statements, Assertion and reason. Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Question. Assertion: Molarity of a solution in liquid state changes with temperature.
Reason: The volume of a solution changes with change in temperature.
Answer
A
Question. Assertion: If a liquid solute more volatile than the solvent is added to the solvent, the vapour pressure of the solution may increase i.e., ps > po.
Reason: In the presence of a more volatile liquid solute, only the solute will form the vapours and solvent will not.
Answer
C
Question. Assertion: If one component of a solution obeys Raoult’s law over a certain range of composition, the other component will not obey Henry’s law in that range.
Reason: Raoult’s law is a special case of Henry’s law
Answer
B
Question. Assertion: When methyl alcohol is added to water, boiling point of water increases.
Reason: When a volatile solute is added to a volatile solvent elevation in boiling point is observed.
Answer
D
Question. Assertion: When NaCl is added to water a depression in freezing point is observed.
Reason: The lowering of vapour pressure of a solution causes depression in the freezing point
Answer
A
CASE BASED QUESTIONS
Read the following passage and answer the questions that follow:
Solutions are homogeneous mixture of two or more substances. Ideal solution follow Raoult’s law. The vapour pressure of each component is directly proportional to their mole fraction if both solute and solvent are volatile. The relative lowering of vapour pressure is equal to mole fraction of solute if only solvent is volatile. Non-ideal solution form azeotropes which cannot be separated by fractional distillation. Henry’s law is special case of Raoult’s law applicable to
gases dissolved in liquids. Colligative properties depend upon number of particles of solute. Relative lowering of vapour pressure, elevation in boiling point, depression in freezing point and osmotic pressure are colligative properties which depend upon mole fraction of solute, molality and molarity of solutions.
Question. 50 ml of an aqueous solution of glucose (Molar mass 180 g/mol) contains 6.02 × 1022 molecules. What is its molarity?
Answer. 6.02 x 1023 molecules → 1 mole of glucose
6.02 x 1022 molecules → 0.1 moles
Molarity = 0.1/0.05 = 2 M
Question. Identify which liquid has lower vapour pressure at 90°C if boiling point of liquid ‘A’ and ‘B’ are 140°C and 180° respectively.
Answer. ‘B’ will have lower vapour pressure because its boiling point is higher.
Question. What type of azeotropes are formed by non-ideal solution showing negative deviation from Raoult’s law?
Answer. Maximum boiling azeotropes.
Question. Why meat is preserved for longer time by salting?
Answer. Salt inhibits the growth of microorganisms by drawing out water from microbial cells through osmosis 20% salt is needed to kill most species of unwanted bacteria.
Question. For a 5% solution of area (molar mass 60 g/mol), calculate the osmotic pressure at 300 K (R = 0.0821 L atm k–1).
Answer. Πv = nRT
Π × 0.1 L = (5/60) × 0.0821 × 300 = 20.52 atm
STATEMENT TYPE QUESTIONS
Question. Define an ideal solution and write one of its characteristics.
Answer. Those solutions which obey Raoult’s law at all concentrations are called ideal solutions. Characteristic of an ideal solution: There will be no change in enthalpy ΔHmix = 0 and volume change on mixing ΔVmix = 0,
Question. Give an example of gaseous solution and liquid solution each.
Answer. Gaseous solution- Water vapour (Liquid in gas) Liquid solution- Ethanol dissolved in water (Liquid in liquid)
Question. State Henry’s law.
Answer. Henry’s law states that “The partial pressure of the gas in vapour phase is proportional to the mole fraction of the gas in the solution”
Question. Define osmotic pressure of a solution.
Answer. It is the external pressure which is applied on the solution side which is sufficient to prevent the entry of the solvent through semi-permeable membrane.
Question. Define Colligative properties.
Answer. All those properties which depend on the number of solute particles in solution irrespective of the nature of solute are called as colligative properties.
TWO MARKS QUESTIONS
Question. H2 S, a toxic gas with rotten egg like smell, is used for the qualitative analysis. If the solubility of H2S in water at STP is 0.195 m, calculate Henry’s law constant.
Answer. Solubility of H2S gas = 0.195 m
= 0.195 mole in 1 kg of solvent 1kg of solvent = 1000g
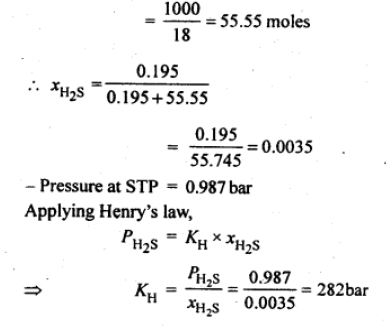
Question. Calculate the mass of urea (NH2CONH2) required in making 2.5 kg of 0.25 molal aqueous solution.
Answer. 0.25 Molal aqueous solution to urea means that moles of urea = 0.25 mole
mass of solvent (NH2CONH2) = 60 g mol-1
.’. 0.25 mole of urea = 0.25 x 60=15g
Mass of solution = 1000+15 = 1015g = 1.015 kg
1.015 kg of urea solution contains 15g of urea
.’. 2.5 kg of solution contains urea =15/1.015 x 2.5 = 37 g
Question. Calculate the mass percentage of benzene (C6H6) and carbon tetrachloride (CCl4) if 22 g of benzene is dissolved in 122 g of carbon tetrachloride.
Answer. Mass of solution = Mass of C6H6 + Mass of CCl4 = 22 g+122 g= 144 g
Mass % of benzene = 22/144 x 100 =15.28 %
Mass % of CCl4 = 122/144 x 100 = 84.72 %
Question. Calculate the mole fraction of benzene in solution containing 30% by mass in carbon tetrachloride.
Answer. 30% by mass of C6H6 in CCl4 => 30 g C6H6 in 100 g solution
.’. no. of moles of C6H6(nC6h6) = 30/78 = 0.385

Question. Calculate the mass of ascorbic acid (vitamin C, C6H8O6) to be dissolved in 75 g of acetic acid to lower its melting point by 1·5°C. (Kf for CH3COOH) = 3·9 K kg mol-1)
Answer.

THREE MARKS QUESTIONS
Question. Calculate the mass of a non-volatile solute(molecular mass 40 g mol-1) that should be dissolved in 114 g of octane to reduce its pressure to 80%.
Answer. According to Raoult’s Law,

Question. Two elements A and B form compounds having formula AB2 and AB4. When dissolved in 20g of benzene (C6H6), 1 g of AB2 lowers the freezing point by 2.3 K whereas 1.0 g of AB4 lowers it by 1.3 K. The molar depression constant for benzene is 5.1 K kg mol-1. Calculate atomic masses of A and B.
Answer.

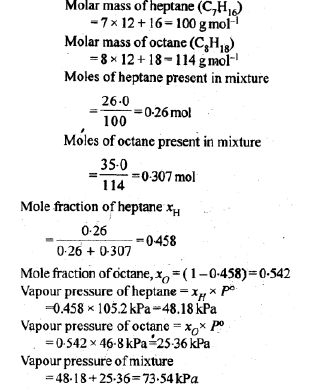
Question. Heptane and octane form an ideal solution. At 373 K, the vapour pressures of the two liquid components are 105.2 kPa and 46.8 kPa respectively. What will be the vapour pressure of a mixture of 26.0 g of heptane and 35.0 g of octane?
Answer.
FIVE MARKS QUESTIONS
Question. (i) State Kohlrausch law.
(ii) Calculate the emf of the following cell at 298 K:
Al(s)/Al3+ (0.15M)//Cu2+(0.025M) /Cu(s)
(Given Eo(Al3+/Al) = -1.66 V, Eo(Cu2+/Cu)=0.34V, log0.15=- 0.8239, log 0.025 = -1.6020)
Answer. (i) limiting molar conductivity of an electrolyte can be represented as the sum of the individual contributions of the anion and cation of the electrolyte.
(ii) Eocell = Eocathode -Eoanode = 0.34-(-1.66) = 2.00 V
Ecell = Eocell – 0.059/n (log [Al3+]2/[Cu2+]3)
Here n = 6
Ecell = 2 – 0.059/6 (log [0.15]2 /[0.025]3 )
= 2 – 0.059/6 ( 2log 0.15 – 3 log 0.025)
= 2 – 0.059/6 (-1.6478 +4.8062) = 2- 0.0311 = 1.9689V
Question. On the basis of Eovalues identify which amongst the following is the strongest oxidising agent
Cl2(g) + 2e– → 2Cl– Eo = +1.36 V,
MnO4– + 8H+ + 5e– → Mn2+ + 4H2O Eo = +1.51 V
Cr2O72– + 14H+ + 6e– → 2Cr3+ + 7H2O Eo = +1.33 V
(ii) The following figure, represents variation of (Λm ) vs √c for an electrolyte. Here Λm is the molar conductivity and c is the concentration of the electrolyte.

a) Define molar conductivity
b) Identify the nature of electrolyte on the basis of the above plot. Justify your answer.
c) Determine the value of Λmo for the electrolyte.
d) Show how to calculate the value of A for the electrolyte using the above graph.
Answer. (i) MnO4–
(ii) (a) Molar conductivity of a solution at a given concentration is the conductance of the volume V of solution containing one mole of electrolyte kept between two electrodes with area of cross section A and distance of unit length.
(b)Strong electrolyte, For strong electrolytes, Λm increases slowly with dilution
(c) Λm = Λm° – Ac1⁄2
Therefore Λm° = 150 Scm2mol–1
(d) A = – slope = – (149 – 147.8/ 0.010-0.022) = 100 Scm2mol–1
