Exam Question for Class 12 Chemistry Chapter 4 Chemical Kinetics
Please refer to below Exam Question for Class 12 Chemistry Chapter 4 Chemical Kinetics. These questions and answers have been prepared by expert Class 12 Chemistry teachers based on the latest NCERT Book for Class 12 Chemistry and examination guidelines issued by CBSE, NCERT, and KVS. We have provided Class 12 Chemistry exam questions for all chapters in your textbooks. You will be able to easily learn problems and solutions which are expected to come in the upcoming class tests and exams for standard 12th.
Chapter 4 Chemical Kinetics Class 12 Chemistry Exam Question
All questions and answers provided below for Exam Question Class 12 Chemistry Chapter 4 Chemical Kinetics are very important and should be revised daily.
Very Short Answer Type Questions (VSA)
Question. If half-life period of a first order reaction is x and 3/4th life period of the same reaction is y, how are x and y related to each other?
Answer.
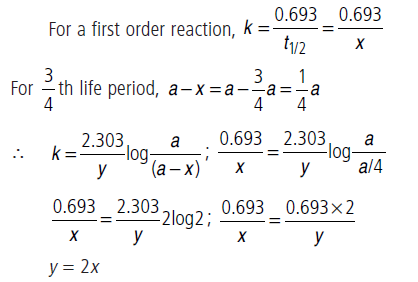
Question. Distinguish between ‘rate expression’ and ‘rate constant’ of a reaction.
Answer.Rate expression is a way of expressing rate of reaction in terms of concentration of reactants, e.g., for a general reaction, aA + bB → cC + dD
Rate = k[A]x [B]y
Rate constant (k) is equal to the rate of reaction when molar concentration of reactant is unity. Its units depends upon the order of reaction.
Question. For a reaction R → P, half-life (t1/2) is observed to be independent of the initial concentration of reactants. What is the order of reaction?
Answer.Half-life of first order reaction is independent of the initial concentration of reactants.
t1/2 = 0.693/k
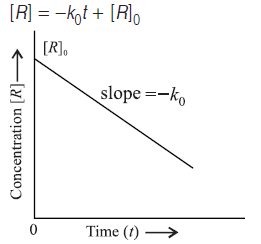
Question. Draw a graph between concentration and time for a zero order reaction.
Answer.
Question. Distinguish between molecularity and order of a reaction.
Answer.

Generally, in a complex reaction the order of reaction is equal to the molecularity of the slowest step.
Question. Express the rate of the following reaction in terms of the formation of ammonia.
N2(g) + 3H2(g) → 2NH3(g)
Answer.

Question. Define the half-life period of reaction (t½).
Answer.The time taken for half of the reaction to complete, i.e., the time in which the concentration of a reactant is reduced to half of its original value is called half-life period of the reaction.

Question. If the rate constant of reaction is k = 3 × 10–4s–1, then identify the order of the reaction.
Answer.First order reaction has s–1 as the unit of the rate constant.
Question. Define elementary step in a reaction.
Answer.Elementary step : Each step of a complex reaction is called an elementary step.
Question. For the reaction 3H2(g) + N2(g) → 2NH3(g),how are the rate of reaction −d[H2]/dt expression and −d[NH3] /dt interrelated?
Answer.
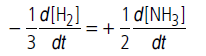
Short Answer Type Questions (SA-I)
Question. For a reaction A + B → P, the rate is given by Rate = k[A][B]2
(i) How is the rate of reaction affected if the concentration of B is doubled?
(ii) What is the overall order of reaction if A is present in large excess?
Answer.(i) From the rate law equation, order of reaction w.r.t. B is 2. Hence, if concentration of B is doubled, rate will become four times.
(ii) If A is present in large excess, rate of reaction will be independent of concentration of A and hence, order of reaction will be 2.
Question. For a chemical reaction R → P, the variation in the concentration [R] vs. time (t) plot is given as
(i) Predict the order of the reaction.
(ii) What is the slope of the curve?

Answer.(i) The reaction is of zero order.
(ii) Slope of the curve = −k = [R]/dt
Question. For a reaction :
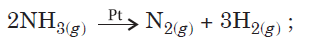
Rate = k
(i) Write the order and molecularity of this reaction.
(ii) Write the unit of k.
Answer.(i) The decomposition of gaseous ammonia on a hot platinum surface is a zero order reaction at high pressure.
In this reaction, platinum metal acts as a catalyst. At high pressure, the metal surface gets saturated with gas molecules.
So, a further change in reaction conditions is unable to alter the amount of ammonia on the surface of the catalyst making rate of the reaction independent of its concentration.
However, two molecules of ammonia react to give products thus, the molecularity is two.
(ii) For a zero order reaction, unit of rate constant is mol L–1 s–1.
Question. For a reaction
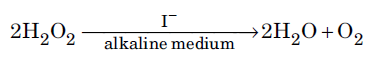
the proposed mechanism is as given below :
(1) H2O2 + I− → H2O + IO− (slow)
(2) H2O2 + IO− → H2O + I− +O2 (fast)
(i) Write rate law for the reaction.
(ii) Write the overall order of reaction.
(iii) Out of steps (1) and (2), which one is rate determining step?
Answer.(i) Rate = k[H2O2][I–]
(ii) Overall order of reaction is 2.
(iii) Step (1) being the slow step is the rate determining step of the reaction.
Question. For the reaction, 2N2O5(g) 4NO2(g) + O2(g), the rate of formation of NO2(g) is 2.8 × 10–3 M s–1.Calculate the rate of disappearance of N2O5(g).
Answer.

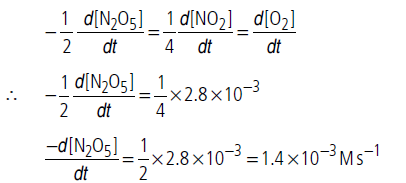
Question. For a first order reaction, show that time required for 99% completion is twice the time required for the completion of 90% of reaction.
Answer.
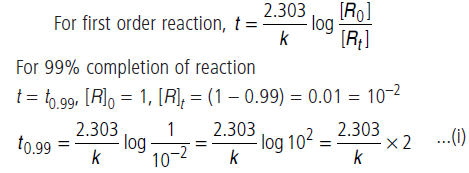

Question. For a reaction A + B → P, the rate law is given by, r = k[A]1/2 [B]2
What is the order of this reaction?
Answer.Rate law, r = k[A]1/2 [B]2
Order of reaction is sum of the powers of concentration terms,
Order of reaction 1/2+2=5/2=2.5
Question. The thermal decomposition of HCO2H is a first order reaction with a rate constant of 2.4 × 10–3 s–1 at a certain temperature. Calculate how long will it take for three-fourth of initial quantity of HCO2H to decompose.
(log 0.25 = – 0.6021)
Answer.

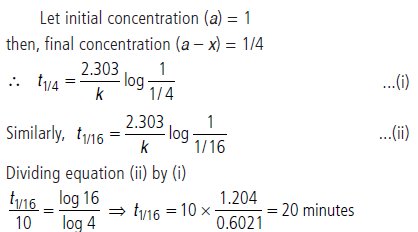
Question. For a first order reaction, the time taken to reduce initial concentration by a factor of 1/4 is 10 minutes. What will be the time required to reduce initial concentration by a factor of 1/16?
Answer.
Question. What is meant by rate of reaction? Differentiate between average rate and instantaneous rate of reaction.
Answer.Change in concentration i.e., either (decrease in concentration of reactant or increase in concentration of product) per unit time is called rate of reaction.
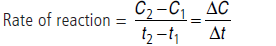
The ratio of change of concentration of reactants to the time consumed in that change is called average rate of reaction.
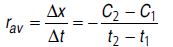
The rate of reaction at a particular instant (time) is called instantaneous rate of reaction.
rins =dx/dt
dx = small change in concentration
dt = small time interval
Short Answer Type Questions (SA-II)
Question. In a pseudo first order hyrolysis of ester in water, the following results are obtained :

(i) Calculate the average rate of reaction between the time interval 30 to 60 seconds.
(ii) Calculate the pseudo first order rate constant for the hydrolysis of ester.
Answer.(i) Average rate of reaction between the time interval 30 to 60 seconds is

Question. For a reaction, the rate law is :
Rate = k [A][B]1/2. Can this reaction be an elementary reaction?
Answer.For an elementary reaction, order should be equal to molecularity and further molecularity should be integral. For the given reaction, order of reaction = 1 + 1/2 = 3/2. Since
molecularity cannot be fractional, therefore, for the given reaction, order is not equal to molecularity. Hence given reaction cannot be an elementary reaction.
Question. What will be the rate of decomposition of N2O5 and rate of formation of NO2 and O2 when [N2O5] = 0.40 M for the reaction 2N2O5 → 4NO2 + O2. The rate constant for this reaction is 3.1 × 10–4 min–1.
Answer.The given reaction is 2N2O5 → 4NO2 + O2
Unit of rate constant suggests that rate of reaction is first order.
Hence, rate of reaction = k[N2O5]
= 3.1 × 10–4 min–1 × 0.40 M
= 1.24 × 10–4 M min–1
Rate of reaction,
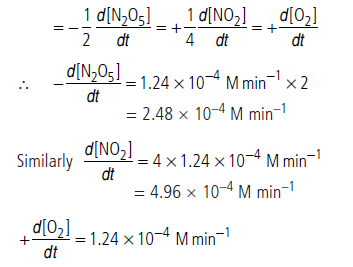
Question. A reaction is first order in A and second order in B.
(i) Write differential rate equation.
(ii) How is rate affected when concentration of B is tripled?
(iii) How is rate affected when concentration of both A and B is doubled?
(iv) What is molecularity of a reaction?
Answer.(i) Differential rate equation of reaction is
dx/dt = k[A]1[B]2 = k[A][B]2
(ii) When conc. of B is tripled, it means conc. of B becomes [3 × B]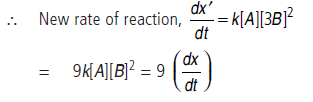
i.e., the rate of reaction will become 9 times.
(iii) When conc. of A is doubled and that of B is also doubled, then conc. of A becomes [2A] and that of B becomes [2B].
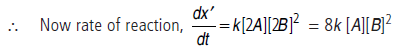
i.e., the rate of reaction will become 8 times.
(iv) Molecularity of a reaction is the number of reacting particles which collide simultaneously to bring about the chemical change. It is a theoretical concept.
Question. The following data were obtained during the first order thermal decomposition of SO2Cl2 at a constant volume :
SO2Cl2(g) → SO2(g) + Cl2(g)
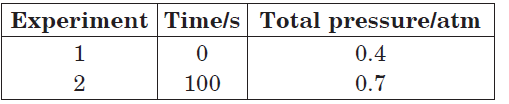
Calculate the rate constant.
(Given : log 4 = 0.6021, log 2 = 0.3010)
Answer.The given reaction is
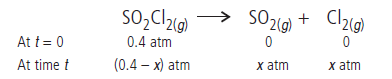
Total pressure at time t will be
Pt = (0.4 – x) + x + x = 0.4 + x
x = (Pt – 0.4)
Pressure of SO2Cl2 at time t will be
pSO2Cl2 = 0.4 – x = 0.4 – (Pt – 0.4) = 0.8 – Pt
At time t = 100 s, Pt = 0.7 atm
pSO2Cl2 = 0.8 – 0.7 = 0.1 atm
According to first order kinetic equation

Question. The rate of decomposition of ammonia is found to depend upon the concentration of NH3 according to the equation
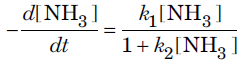
What will be the order of reaction when
(i) concentration of NH3 is very high?
(ii) concentration of NH3 is very low?
Answer.The given rate law equation can be written as
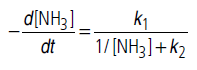
(i) If [NH3] is very high, 1/[NH3] becomes negligible
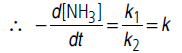
i.e., rate becomes independent of concentration. Hence, it is of zero order.
(ii) If [NH3] is is very small, 1/[NH3] will be very large
(>>k2), so that k2 can be neglected in comparison to 1/[NH3]. Hence
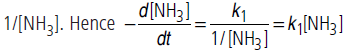
Thus, reaction is of 1st order.
Question. From the data given below, calculate order of reaction.

Answer.


Question. What do you understand by the ‘order of a reaction’? Identify the reaction order from each of the following units of reaction rate constant :
(i) L–1 mol s–1 (ii) L mol–1 s–1
Answer.Order of a reaction : It is the sum of the power of reactant in the rate law expression.
(i) L–1 mol s–1 – Zero order reaction
(ii) s–1 – First order reaction
Question. Following data are obtained for the reaction :
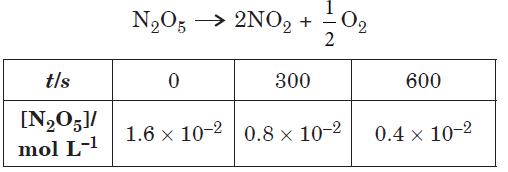
(a) Show that it follows first order reaction.
(b) Calculate the half-life.
(Given : log 2 = 0.3010, log 4 = 0.6021)
Answer.(a) The formula of rate constant for first order reaction is


Question. For the first order thermal decomposition
reaction, the following data were obtained :
C2H5Cl(g) → C2H4(g) + HCl(g)
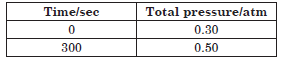
Calculate the rate constant.
(Given : log 2 = 0.301, log 3 = 0.4771, log 4 = 0.6021)
Answer.

Question. Hydrogen peroxide, H2O2(aq) decomposes to H2O(l) and O2(g) in a reaction that is first order in H2O2 and has a rate constant k = 1.06 × 10–3 min–1.
(i) How long will it take for 15% of a sample of H2O2 to decompose?
(ii) How long will it take for 85% of the sample to decompose?
Answer.

Question. For a chemical reaction R → P, the variation in the concentration, ln [R] vs. time (s) plot is given as
(i) Predict the order of the reaction.
(ii) What is the slope of the curve?
(iii) Write the unit of the rate constant for this reaction.
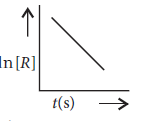
Answer.(i) The reaction is of 1st order.
(ii) For first order reaction ln[R] = –kt + ln [R]0
comparing eqn. y = m × x + c
we get a straight line with slope = –k and intercept equal to ln[R]0.
(iii) Unit of rate constant for first order reaction
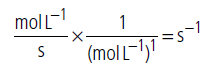
Question. When inversion of sucrose is studied at pH = 5, the half-life period is always found to be 500 minutes irrespective of any initial concentration but when it is studied at pH = 6, the half-life period is found to be 50 minutes. Derive the rate law expression for the inversion of sucrose.
Answer.At pH = 5, as half-life period is found to be independent of initial concentration of sucrose, this means with respect to
sucrose, it is a reaction of first order, i.e., Rate = k[Sucrose].
If n is the order with respect to H+ ion, t1/2 ∝[H+]1–n,
i.e., 500 ∝ (10–5)1 – n [pH = 5 means [H+] = 10–5 M] …(i)
and 50 ∝ (10–6)1 – n [pH = 6 means [H+] = 10–6M] …(ii)
Dividing (i) by (ii), 10 = (10)1 – n i.e. 1 – n = 1 or n = 0, i.e.,order with respect to H+ ion = 0. Hence, overall rate law is
Rate = k [Sucrose] [H+]0.
Question. A first order reaction takes 160 minutes time for 20% completion. Calculate time required for half completion of reaction.
Answer.

Question. A certain reaction takes 5 minutes for initial concentration 0.5 mol L–1 to become 0.25 mol L–1 and another 5 minutes to becomes 0.125 mol L–1.What is the order and specific rate constant of the reaction?
Answer.The given data is
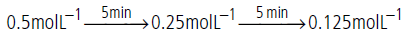
Half life period is independent of initial concentration of the reactant, hence reaction is of first order.
For first order reaction
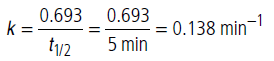
Long Answer Type Questions
Question. The half-life period of a first order reaction is 30 minutes. Calculate the specific reaction rate of the reaction. What fraction of the reactant remains after 70 minutes?
Answer.

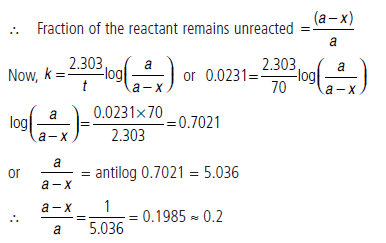
Question. For the hydrolysis of methyl acetate in aqueous solution, the following results were obtained :

(i) Show that it follows pseudo first order reaction, as the concentration of water remains constant.
(ii) Calculate the average rate of reaction between the time interval 30 to 60 seconds.
(Given : log 2 = 0.3010, log 4 = 0.6021)
Answer.

Question. The following results have been obtained during the kinetic studies of the reaction :
2A + B →C + D
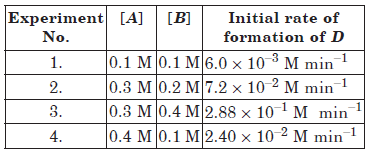
Calculate the rate of formation of D when
[A] = 0.5 mol L–1 and [B] = 0.2 mol L–1.
Answer.Suppose order with respect to A is m and with respect to B is n. Then the rate law will be
Rate = k[A]m[B]n
Substituting the value of experiments 1 to 4, we get
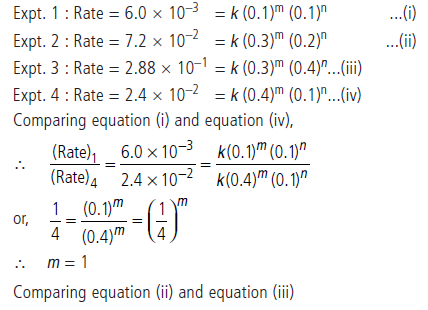
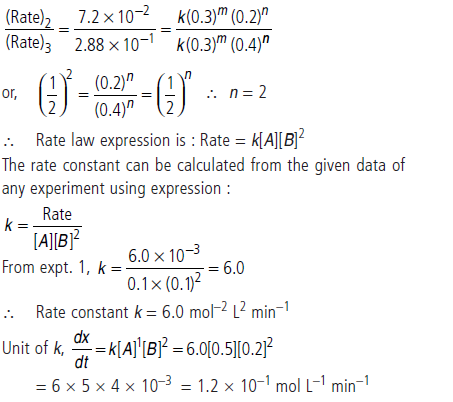
Question. Calculate the order of the reaction and the rate constant for the decomposition of N2O5 at 30°C from the following rate data.
Answer.
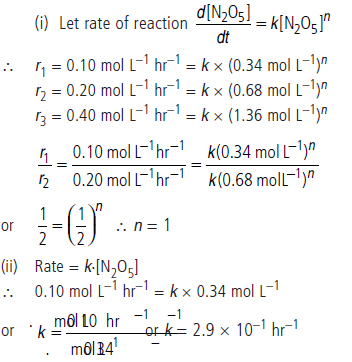
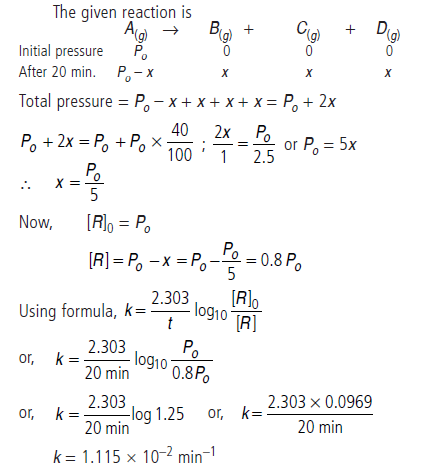
Question. For a homogeneous gas phase reaction
A(g) → B(g) + C(g) + D(g), the pressure of the reaction mixture increases by 40% in 20 minute.
Calculate rate constant of a reaction.
Answer.