Exam Question for Class 12 Chemistry Chapter 5 Surface Chemistry
Please refer to below Exam Question for Class 12 Chemistry Chapter 5 Surface Chemistry . These questions and answers have been prepared by expert Class 12 Chemistry teachers based on the latest NCERT Book for Class 12 Biology and examination guidelines issued by CBSE, NCERT, and KVS. We have provided Class 12 Chemistry exam questions for all chapters in your textbooks. You will be able to easily learn problems and solutions which are expected to come in the upcoming class tests and exams for standard 12th.
Chapter 5 Surface Chemistry Class 12 Chemistry Exam Question
All questions and answers provided below for Exam Question Class 12 Chemistry Chapter 5 Surface Chemistry are very important and should be revised daily.
Very Short Answer Type Questions (VSA)
Question. Name of the temperature above which the formation of micelles takes place.
Answer.The formation of micelles takes place only above a particular temperature called Kraft temperature (Tk).
Question. Based on the type of dispersed phase, what type of colloid is micelles?
Answer.Associated colloids.
Question. Why are medicines more effective in colloidal state?
Answer.Medicines are more effective in colloidal form because in this form, these are more easily assimilated due to large surface area.
Question. A colloidal sol is prepared by the given method in figure.What is the charge on hydrated ferric oxide colloidal particles formed in the test tube? How is the sol represented?

Answer.FeCl3 + NaOH → Fe2O3·xH2O/OH–
Negatively charged sol
Question. How can a colloidal solution and true solution of the same colour be distinguished from each other.
Answer. When a powerful beam of light is passed through colloidal solution it exhibits tyndall effect whereas true solution does not.
Question. Give reasons for the following observations:
A delta is formed at the meeting point of sea water and river water.
Answer.Sea water contains electrolytes. River contains colloids of sand and clay. When they meet the electrolytes neutralise the charge on colloidal particles which results in the precipitation of sand, clay etc. thus, resulting in a delta formation.
Question. Physisorption is multi-layered, while chemisorption is mono-layered. Explain.
Answer.In physisorption, the gas can be adsorbed one over the other by van der Waals’ forces, thus is multilayered while chemisorpiton involves formation of a chemical bond, which
can be formed only with the layer that is in direct contact with the adsorbent. Therefore, it is mono-layered.
Question. Out of NH3 and CO2, which gas will be adsorbed more readily on the surface of activated charcoal and why?
Answer.NH3 gas will be adsorbed more readily on the surface because it has higher critical temperature than CO2 gas.
Question. Explain the following :
Artificial rain is caused by spraying salt over clouds.
Answer.Clouds are aerosols and the water particles in air carry some charge over them. Rainfall can occur when two oppositely charged clouds meet. Spraying a sol carrying charge opposite to the one on clouds causes artificial rain.
Question. Out of BaCl2 and KCl, which one is more effective in causing coagulation of a negatively charged colloidal sol? Give reason.
Answer.BaCl2 is more effective in causing coagulation of negatively charged colloidal sol.
Because greater the valency of the coagulating ion, greater is its power to bring about coagulation.
Short Answer Type Questions (SA-I)
Question. Give reasons for the following observations:
(i) Leather gets hardened after tanning.
(ii) Lyophilic sol is more stable than lyophobic sol.
Answer.(i) Animal hides are colloidal in nature. When a hide,which has positively charged particles is soaked in tannin, containing negatively charged colloidal particles, mutual
coagulation takes place. This results in the hardening of leather.
(ii) Lyophilic sol is more stable than lyophobic sol because it is highly hydrated in the solution.
Question. Pressure of a closed vessel filled with gas decreases when powdered charcoal is added. Explain.
Answer. Powdered charcoal is a good adsorbent. It adsorbs the gases on its surface which reduces the pressure of the gas in the enclosed vessel.
Question. (i) What is the role of activated charcoal in gas mask?
(ii) How does chemisorption vary with temperature?
Answer.(i) Activated charcoal in gas masks adsorb the poisonous gases present in air and thus purify the air for breathing.
(ii) Effect of temperature : Chemisorption is an exothermic process but is very slow at lower temperature. High temperature is more favourable thus with increasing temperature, rate of adsorption increases.
Question. Give reasons for the following observations :
(i) NH3 gas absorbs more readily than N2 gas on the surface of charcoal.
(ii) Powdered substances are more effective adsorbents.
Answer.(i) Higher the critical temperature of the gas, more readily it can get adsorbed on the surface of an adsorbent since van der Waals’ forces are stronger at this temperature.
NH3 (132°C) has a higher critical temperature than dinitrogen (–147°C) thus, it gets adsorbed more readily than N2.
(ii) A finely divided substance is more effective as adsorbent because it has more surface area and more number of active sites (active centres) which increases the extent of adsorption.
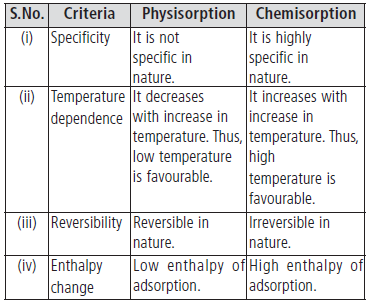
Question. Write the differences between physisorption and chemisorption with respect to the following:
(i) Specificity (ii) Temperature dependence
(iii) Reversibility and (iv) Enthalpy change
Answer.
Question. (i) Out of MgCl2 and AlCl3, which one is more effective in causing coagulation of negatively charged sol and why?
(ii) Out of sulphur sol and proteins, which one forms multimolecular colloids?
Answer.(i) According to Hardy-Schulze rule, for a negatively charged sol, greater is the valency of the positive ion added, greater is its coagulation power.
Thus, AlCl3 (Al3+) is more effective than MgCl2(Mg2+) in causing coagulation of negatively charged sol.
(ii) Proteins are macromolecules which cannot form multimolecular colloids while sulphur sol have smaller S8 molecules which can form multimolecular colloids.
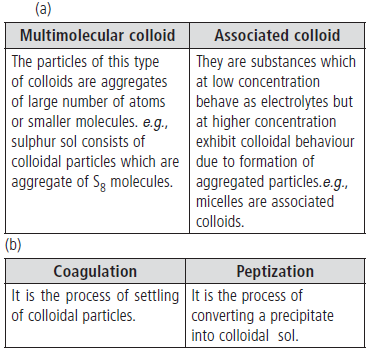
Question. What is meant by coagulation of a colloidal solution? Name any method by which coagulation of lyophobic sols can be carried out.
Answer.Coagulation : The process of aggregating together of colloidal particles into large sized particle which ultimately settles down under the force of gravity as a precipitate is
called coagulation.
Coagulation of lyophobic sols can be carried out by adding electrolyte.
Question. Write one difference in each of the following:
(a) Multimolecular colloid and associated colloid
(b) Coagulation and peptization
Answer.
Question. What happens when
(a) a freshly prepared precipitate of Fe(OH)3 is shaken with a small amount of FeCl3 solution?
(b) persistent dialysis of a colloidal solution is carried out?
Answer.(a) On treating a precipitate of iron (III) hydroxide with a small amount of FeCl3 solution, a reddish brown coloured colloidal solution is formed. In this case, Fe3+ ions from ferric chloride are adsorbed by Fe(OH)3 precipitate.
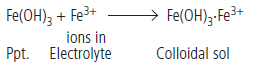
(b) When dialysis is persistent and prolonged, traces of electrolyte are also removed. These electrolytes stabilise the colloid and when removed completely, the unstable colloid gets coagulated.
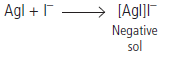
Question. (i) Same substances can act both as colloids and crystalloids. Explain.
(ii) What will be the charge on AgI colloidal sol when it is prepared by adding small amount of AgNO3 solution to KI solution in water? What is responsible for the development of this charge?
Answer.(i) When the size of the particles lies between 1 to 1000 nm, it behaves as a colloid. If particle size is less than 1 nm, it exists as a true solution and behaves as a crystalloid.
(ii) When AgNO3 solution is added to aqueous KI solution, a negatively charged sol of AgI is formed. This is due to selective adsorption of I– ions from the dispersion medium on AgI.
Short Answer Type Questions (SA-II)
Question. Give reasons for the following :
(a) Brownian movement provides stability to the colloidal solution.
(b) True solution does not show Tyndall effect.
(c) Addition of alum purifies water.
Answer.(a) The Brownian movement has a stirring effect which does not permit the particles to settle and thus, it is responsible for the stability of colloidal solutions.
(b) Tyndall effect is not observed in true solutions as the diameter of dispersed particles is very small to disperse the light incident on it.
(c) The water obtained from natural sources often contains suspended impurities. Alum is added to coagulate the suspended impurities and make the water fit for drinking purposes.
Question. (i) Write the dispersed phase and dispersion medium of the following colloidal systems :
(a) Smoke (b) Milk
(ii) In reference to Freundlich adsorption isotherm write the expression for adsorption of gases on solids in the form of an equation.
Answer.(i) (a) Dispersed phase of smoke = Solid
Dispersion medium of smoke = Gas
(b) Dispersed phase of milk = Fat (liquid)
Dispersion medium of milk = Water (liquid)

where, x is the mass of gas adsorbed on mass m of the adsorbent at pressure p.
Question. Distinguish between multimolecular, macromolecular and associated colloids. Give one example of each.
Answer.Multimolecular colloids : When a large number of small molecules or atoms of a substance combine together in a dispersion medium to form aggregates, having size in the colloidal range, the colloidal solutions thus, formed are known as multimolecular colloids. e.g., sulphur sol.
Macromolecular colloids: When macromolecules which have large molecular masses are dispersed in a suitable dispersion medium, they form a solution in which the size
of the macromolecule may be in the colloidal range. Such colloids are called macromolecular colloids. e.g., cellulose,starch, etc.
Associated colloids : They are substances which at low concentration behave as electrolytes but at higher concentration exhibit colloidal behaviour due to formation of aggregated particles. e.g., micelles are associated colloids.
Question. Define the following terms :
(i) Lyophilic colloid
(ii) Zeta potential
(iii) Associated colloids
Answer.(i) A colloidal sol in which dispersed phase and dispersion medium attract each other is called lyophilic colloid. e.g., gum.
(ii) The difference in potential between the fixed layer and the diffused layer of opposite charges in a colloidal sol is known as electrokinetic or zeta potential.
(iii) Associated colloids : Micelles are associated colloids.
They are substances which at low concentrations behave as strong electrolytes but at higher concentrations exhibit colloidal behaviour due to formation of aggregates.
Question. Explain what is observed when :
(i) A beam of light is passed through a colloidal solution.
(ii) NaCl solution is added to hydrated ferric oxide sol.
(iii) Electric current is passed through a colloidal solution.
Answer.(i) Scattering of light by the colloidal particles takes place and the path of light becomes visible (Tyndall effect).
(ii) The positively charged colloidal particles of ferric hydroxide sol get coagulated by the oppositely charged Cl– ions provided by NaCl.
(iii) On passing electric current through a sol, colloidal particles start moving towards oppositely charged electrodes where they lose their charge and get coagulated (electrophoresis).
Question. What is meant by coagulation of a colloidal solution? Describe briefly any three methods by which coagulation of lyophobic sols can be carried out.
Answer.The process of settling of colloidal particles is called coagulation of the sol. It is also known as precipitation.
Following are the three methods by which coagulation of lyophobic sols can be carried out :
(i) Electrophoresis : In this process, the colloidal particles move towards oppositely charged electrodes and get discharged resulting in coagulation.
(ii) Mixing of two oppositely charged sols : When equal proportions of oppositely charged sols are mixed, they neutralise each other resulting in coagulation.
(iii) Persistent dialysis : On prolonged dialysis, electrolytes present in sol are removed completely and colloids become unstable resulting in coagulation.
Question. (i) How are the following colloidal solutions prepared?
(a) Sulphur in water
(b) Gold sol
(ii) Why is adsorption always exothermic?
Answer.
i) (a) Sulphur sol is prepared by the oxidation of H2S with SO2.
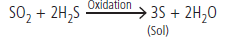
(b) Gold sol is prepared by Bredig’s arc process or by the reduction of AuCl3 with HCHO.

(ii) In adsorption, there is always a decrease in residual unbalanced forces on the surface. This results in decrease in surface energy which appears as heat. Hence, adsorption is unconditionally an exothermic process.
Question. Classify colloids where the dispersion medium is water. State their characteristics and write an example of each of these classes.
Answer.(i) Sol : When solid is dispersed in water, it is called sol, e.g., gold sol.
(ii) Emulsion: When liquid is dispersed in water, it is called emulsion, e.g., milk.
(iii) Foam : When gas is dispersed in water, it is called foam, e.g., soap lather, whipped cream.
Question. (i) What is the principle of separation of inert gases from its mixture?
(ii) Why silica and alumina gels are used for removing moisture and controlling humidity?
(iii) How does adsorption of a gas on a solid surface vary with temperature?
Answer.
(i) The separation of inert gases from a mixture is based on the difference in degree of adsorption of gases by the coconut charcoal.
(ii) Alumina and silica are good adsorbents. They can adsorb even small amount of moisture present in atmosphere.
(iii) Adsorption of gas on solid surface decreases with rising temperature.
Question. (i) Explain the following terms giving one example for each.
(a) Micelles
(b) Aerosol
(ii) Write one similarity between physisorption and chemisorption.
Answer.(i) (a) Aggregated particles of associated colloids at high colloidal concentration are called micelles. e.g., soaps.
(b) Colloid of a liquid in a gas is called aerosol e.g., fog, etc.
(ii) Physical adsorption and chemical adsorption both increase with increase in surface area of the adsorbent.
Question. (i) Of physisorption and chemisorption,which has a higher enthalpy of adsorption?
(ii) Physisorption is reversible while chemisorption is irreversible. Why ?
(iii) What type of forces are responsible for the occurrence of physisorption?
Answer.(i) Chemisorption has higher enthalpy of adsorption.
(ii) Physisorption takes place on account of weak van der Waals’ forces and no chemical bond is formed, thus the process is reversible. Chemisorption, on the other hand,
involves compound formation, thus it is irreversible in nature.
(iii) The forces operating in physisorption are weak van der Waals’ forces.
Question. (i) Define the following terms giving an example: Hydrosol
(ii) Which complex ion is formed when undecomposed AgBr is washed with hypo solution in photography?
Answer.(i) Hydrosol : It is a colloidal solution in which water is the dispersion medium. e.g., starch solution.
(ii) The developed film is immersed in sodium thiosulphate
(hypo) solution which removes uncharged silver bromide as a complex ion. This is known as fixing.
AgBr + 2Na2S2O3 → Na3[Ag(S2O3)2] + NaBr
After fixing, the film is not sensitive to light.
Question. Giving appropriate examples, explain how the two types of processes of adsorption (physisorption and chemisorption) are influenced by the prevailing temperature, the surface area of adsorbent and the activation energy of the process?
Answer.Effect of temperature : Physisorption decreases with increase of temperature and chemisorption first increases then decreases with increase of temperature.
Surface area : Greater the surface area, greater is the extent of physisorption and chemisorption.
Activation energy : In physisorption, no appreciable activation energy is needed. In chemisorption, sometimes high activation energy is needed.
Question. (i) What are protective colloids? Which type of colloids are used as protective colloids?
(ii) Why does sky look blue?
Answer.(i) The colloids which protect coagulation of other colloids from the electrolytes are called protective colloids.Lyophilic colloids are used as a protective colloid for lyophobic
colloids.
(ii) Dust particles along with water suspended in air have size smaller than wavelength of visible light and are more effective in scattering light of shorter wavelength, blue light
which has smallest wavelength reaches our eyes and the sky looks blue to us.
Question. Ranju is using normal water for washing clothes. She observed that her clothes were not getting very clean although she is using more amount of soaps or detergents. Her friend Swarna advised Ranju washing clothes in warm water. Ranju was surprised to see that washing of clothes with soaps or detergents is easier in luke warm water than cold water.
Now answer the following questions:
(i) What are the processes involved in washing of clothes?
(ii) Why washing of clothes using soap or detergent is easier in warm water?
Answer.(i) Washing of clothes involves micelle formation and emulsification.
(ii) Washing of clothes is due to micelle formation. Micelles are formed at a certain minimum temperature known as Kraft’s temperature. This temperature is more readily achieved in warm water as compared to cold water.
Long Answer Type Questions (LA)
Question. What is an adsorption isotherm? Describe Freundlich adsorption isotherm.
Answer.Adsorption isotherm : It is the variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature.
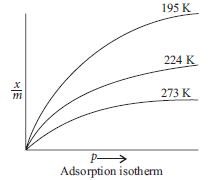
These curves indicate that on increasing temperature, physical adsorption decreases at a fixed pressure.
Freundlich adsorption isotherm : It is an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature.
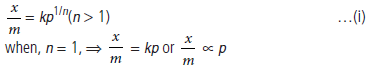
where, x is the mass of gas adsorbed on mass m of the adsorbent at pressure p. k and n are constants which depend on the nature of the adsorbent and the gas at the particular temperature.
Taking log in Eq. (i), gives

The validity of Freundlich isotherm can be verified by plotting x/m on y-axis and log p on x-axis.
If it comes to be a straight line, the Freundlich isotherm is valid.

Question. (i) 1 g of charcoal adsorbs 100 mL of 0.5 M CH3COOH to form a monolayer, and thereby the molarity of CH3COOH reduces to 0.49 M. Calculate the surface area of the charcoal adsorbed by each molecule of acetic acid. Surface area of charcoal
= 3.01 × 102 m2.
(ii) A solution of palmitic acid (M = 256g) in benzene contains 4.24 g acid per litre. When this solution is dropped on the water surface, benzene evaporates and palmitic acid forms monomolecular film of the solid type. If we wish to cover an area of 500 cm2 with a monolayer,what volume of solution should be used? The area occupied by one palmitic acid molecule may be taken to be 21 × 10–20 m2.
Answer.
(i) Number of moles of acetic acid in 100 mL before adding charcoal = 0.05
Number of moles of acetic acid in 100 mL after adding charcoal = 0.049
Number of moles of acetic acid adsorbed on the surface of
charcoal = (0.05 – 0.049) = 0.001
Number of molecules of acetic acid adsorbed on the surface of charcoal = 0.001 × 6.02 × 1023 = 6.02 × 1020
Surface area of charcoal = 3.01 × 102 m2
Area occupied by one molecule of acetic acid on the surface of charcoal
Question. (a) Assuming adsorption to be a spontaneous process, show thermodynamically that it is always an exothermic process.
(b) Why are all adsorption processes exothermic?
(c) How is the adsorption of a gas related to its critical temperature?
(d) A small amount of silica gel and a small amount of anhydrous calcium chloride are placed separately in two corners of a vessel containing water vapour. What phenomenon will occur?
Answer.(a) For spontaneous process, free energy decreases i.e.,
ΔG = –ve.
During adsorption process, the gas is adsorbed on the surface of adsorbent, hence it involves the loss of degree of freedom of the gas, therefore, entropy should also decrease during this process i.e., ΔS = –ve.
Now from ΔG = ΔH – TDS
for adsorption –ΔG = ΔH – [T(–ΔS)] = ΔH + TΔS
That means DG can be negative if ΔH has sufficiently high negative value, hence the process is exothermic (ΔH = –ve).
(b) In adsorption, there is always a decrease in residual unbalanced forces on the surface. This results in decrease in surface energy which appears as heat. Hence, adsorption is
always an exothermic process.
(c) The amount of a gas adsorbed by solid depends on the nature of the gas. In general, higher the critical temperature of a gas, greater is the ease of liquefaction of gas i.e., larger
are the van der Waals’ forces of attraction. Therefore, greater is the adsorption.
(d) Silica gel and calcium chloride are good adsorbents.
Therefore, adsorption of water vapours will occur in both the cases. They will desiccate the vessel completely.
Question. (a) Give reason, why a finely divided substance is more effective as an adsorbent?
(b) Physical and chemical adsorptions respond differently to rise in temperature. What is this difference and why is it so?
(c) The volume of nitrogen gas at 0°C and 1.013 bar required to cover a sample of silica gel with unimolecular layer is 129 cm3 g–1 of gel. Calculate the surface area per gram of the gel if each nitrogen molecule occupies
16.2 × 10–20 m2.
Answer.(a) Adsorption of an adsorbate increases with increasing surface area of adsorbent. Since surface area of a finely divided substance is larger than any other form of adsorbent,
hence it is more effective as adsorbent.
(b) Adsorption isobar for physical adsorption shows that the extent of adsorption decreases with the increase in temperature. The adsorption isobar of chemical adsorption shows that the extent of adsorption first increases and then decreases with the increase in temperature. The initial increase in the extent of adsorption with temperature is due to the fact that the heat supplied acts as activation energy required for chemical adsorption which is not required for physical adsorption.

Question. (i) Explain why excessive dialysis should be avoided for purification of a colloid?
(ii) What is the difference between dialysis and ultrafiltration?
Answer.(i) Dialysis is the process of removing the electrolyte particles from the colloidal solution by means of diffusion through semi-permeable membrane. The charged nature of the ‘sol’ is due to ions of the electrolyte adsorbed, which makes it stable. If the electrolyte is completely removed from the sol by excessive dialysis, then the uncharged particles will come nearer to each other and coagulate resulting in precipitation of the ‘sol’. Therefore excessive dialysis should be avoided.
(ii) The colloidal particles can’t pass through semi-permeable membrane, therefore electrolytes or other molecules from a ‘sol’ can be separated by diffusing through the membrane. This process of purification of a ‘sol’ is known as dialysis. One of the application of dialysis is purification of blood using artificial kidney machine. The separation of ‘sol’ particles from the liquid medium as well as from electrolytes by filtration through an ultrafilter paper such as cellophane is called as ultrafiltration. After separation of ‘sol’ particles it can be further mixed with the dispersion medium to get pure ‘sol’.
