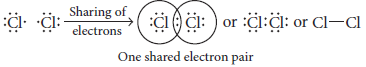Sample Paper Class 10 Science Term 2 Set C
Please refer to Sample Paper Class 10 Science Term 2 Set C with solutions provided below. We have provided CBSE Sample Papers for Class 10 Science as per the latest paper pattern and examination guidelines for Standard 10 Science issued by CBSE for the current academic year. The below provided Sample Guess paper will help you to practice and understand what type of questions can be expected in the Class 10 Science exam.
CBSE Sample Paper Class 10 Science for Term 2 Set C
SECTION – A
1. What is meant by periodicity of properties of elements? Why are the properties of elements placed in the same group of the periodic table similar?
Ans. When elements are arranged in increasing order of their atomic numbers, elements with similar chemical properties are repeated at definite intervals. This is known as periodicity of properties of elements.
Elements placed in the same group of the periodic table have similar properties because they have same number of outermost electrons and hence, show same valency. Thus, they all will form similar type of compounds.
2. Rewrite the following statements after correction, giving reasons.
(i) Elements in the same period have equal valency.
(ii) The metallic character of elements in a period increases gradually on moving from left to right.
Ans. (i) Elements in the same group have equal valency because the elements of the same group have same number of valence electrons.
(ii) The metallic character of elements in a period decreases gradually on moving from left to right because the tendency to lose electrons (electropositivity) decreases on moving from left to right in a period.
OR
In the periodic table, how does the tendency of atoms to lose electrons change on going from
(i) left to right across a period?
(ii) top to bottom in a group?
Ans. (i) Tendency of atoms to lose electrons decreases from left to right in a period.
(ii) Tendency of atoms to lose electrons increases down the group.
3. Identify labelled part A- D and write their functions.
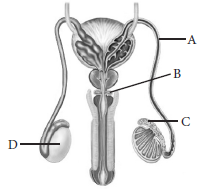
Ans. In the given figure, part ‘A’ is vas deferens that carries sperms. Part ‘B’ is Cowper’s glands that secrete fluid which helps in the lubrication of the penis during copulation. Part ‘C’ is epididymis that stores sperms and part ‘D’ is testis that produces sperms and testosterone.
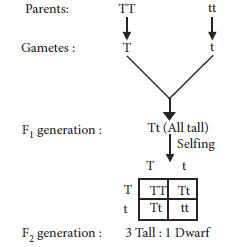
4. How did Mendel interpret his result to show that traits may be dominant or recessive? Describe briefly.
Ans. Mendel crossed the pea plant for two contrasting characters under consideration. The trait that expressed itself in F1 generation was dominant and the one not expressed in F1 generation was recessive. He later selfed the plants of F1 generation and recovered, both parental traits in a definite proportion in F2 generation. Mendel interpreted his results as, the trait that expressed itself in F1 was dominant and the one that reappeared in F2 generation was recessive. It can be demonstrated by the following cross :
5. What will happen if there is no greenhouse effect?
Ans. Greenhouse gases like CO2, CH4 and CFCs are essential for keeping the earth warm and hospitable. They prevent a substantial part of long wave radiations emitted by earth to escape into space, but these gases radiate a part of this energy back to the earth. This phenomenon is called greenhouse effect. Because of greenhouse effect, the mean annual temperature of earth is 15°C. In its absence, it will fall to – 18°C.
OR
Why the flow of energy in food chain is unidirectional?
Ans. A food chain consists of series of populations which are related by eating and being eaten. There is a unidirectional flow of energy from sun to producers and subsequently to series of different types of consumers, i.e.,
Solar radiations → Producers → Herbivores → Carnivores
It cannot pass in reverse direction. There is always a decrease in the flow of energy and content with rise in trophic level. As some energy is lost at each step as heat and is also used up in various metabolic activities.
6. (a) LPG (liquefied petroleum gas) is used as a fuel for cooking in homes. Why is LPG stored as a liquid?
(b) Which among the following has shortest bond length : C2H4, C2H6, C2H2
Ans. (a) LPG is stored as a liquid so that more fuel can be kept in a container. Liquids contain more particles per unit volume than gases. It is also easier to transport liquids than gases.
(b) HC ≡ CH(C2H2) has shortest bond length.
OR
(a) Why carbon does not form the ionic compounds?
(b) Draw the electron dot structure of ethane.
Ans. (a) For formation of ionic compounds, carbon should either gain four electrons to form C4– ions or should lose four electrons to form C4+ ions but formation of both these ions is not possible due to high energy considerations.
(b) Molecular formula of ethane is C2H6. Its electron dot structure is :

7. A copper wire has diameter 0.5 mm and resistivity 1.6 × 10–8 Ω m. Calculate the length of this wire to make it resistance 100 W. How much does the resistance change if the diameter is doubled without changing its length?
Ans. Given; resistivity of copper wire = 1.6 × 10–8 Ω m,
diameter of wire, d = 0.5 mm
and resistance of wire, R = 100 Ω
Radius of wire, r = d / 2 = 0.5 / 2 mm
= 0.25 mm = 2.5 ×10–4 m
Area of cross-section of wire, A = πr2
∴ A = 3.14 × (2.5 × 10–4)2
≅ 1.9625 × 10–7 m2
≅ 1.9 × 10–7 m2
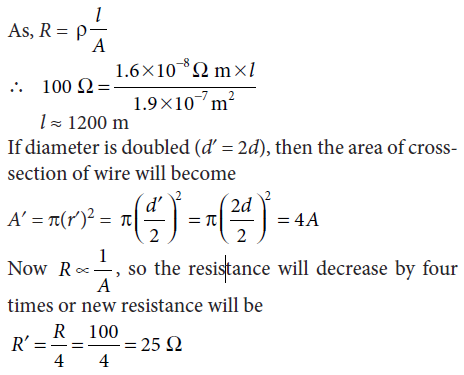
SECTION – B
8. How reproduction takes place in the following organisms?
(a) Hydra
(b) Planaria
(c) Bryophyllum
Ans. (a) In Hydra, a bud develops as an outgrowth due to repeated cell division at one specific site which develops into tiny individuals and detach from the parent body on maturation and become new independent individuals.
(b) Planaria can be cut into any number of pieces and each piece grows into a complete organism. This process is known as regeneration.
(c) Buds, produced in the notches along the leaf margin of Bryophyllum, fall on the soil and develop into new plants.
OR
What is the significance of sexual mode of reproduction?
Ans. Sexual reproduction may be defined as the production of offsprings (new individuals) by the fusion of two gametes (usually one from male parent and the other from female parent) to form a diploid zygote which develops into a mature organism. Gamete formation involves meiosis or reduction division. The gamete mother cell is diploid (2n), i.e., it has two sets of chromosomes. This single diploid cell divides by meiosis to form 4 haploid (n) daughter cells. Each daughter cell becomes a gamete, either male or female.
Each gamete possesses single set of chromosomes. Thus, this division involves copying of the DNA as well as the cellular apparatus. There is a stage in such nuclear division where crossing over of chromosomes take place. This is very important step which results in a slight different composition of chromosomes in gametes. Fusion of these gametes results in the formation of a slightly different individuals which show variations. The variations which lead to the appearance of such characters which fit to the changing environment result in the survival of the species. Chances of variation, therefore, are much more in sexual mode of reproduction as compared to asexual reproduction. Moreover, chances of the production of compatible generations are also more in sexual reproduction.
9. Write a note on vegetative propagation.
Ans. In vegetative propagation, a new plant can be obtained from vegetative parts such as stem, root and leaves. Vegetative propagation property can be used in growing more plants by different methods like:
(i) Layering, e.g., jasmine plant
(ii) Cutting method, e.g., sugarcane, rose, grapevine
(iii) Grafting method, e.g., roses, mango, citrus fruit plants, etc.
Vegetative propagation is advantageous as-
(i) it is a fast, convenient and economical method to produce many plants of commercial importance.
(ii) plants which do not have capacity to produce seeds can be produced through it.
(iii) it produces plants identical to its parent plant with respect to various traits.
10. Two circular coils A and B are kept close to each other, of which coil A carries a current. What will you observe in the galvanometer connected across the coil B
(a) if current in the coil A is changed?
(b) if both the coils are moved in the same direction with the same speed? Give reason to justify your answer in each case.
Ans. (a) When the amount of current in the coil A is changed, an induced current will induce in the coil B due to change in magnetic field lines i.e., magnetic flux.
(b) If both the coils are moved in the same direction with the same speed, then there is no net change in magnetic flux. Hence there will be no deflection in the galvanometer.
11. Write Joule’s law of heating.
Ans. The Joule’s law of heating implies that heat produced in a resistor is
(i) directly proportional to the square of current for a given resistance,
(ii) directly proportional to resistance for a given current, and
(iii) directly proportional to the time for which the current flows through the resistor.
i.e., H = I2 Rt
OR
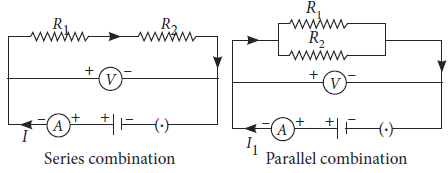
Two resistors are connected first in series and then in parallel across a battery as shown. What effect will it have on the readings of voltmeter and ammeter?
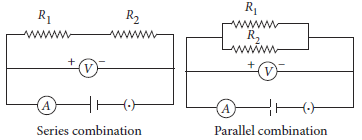
Ans. There will be no change in the reading of voltmeter because applied voltage remains the same in both the cases but ammeter reading will be less in case of series combination as compared to parallel combination of the same resistors because Rs > Rp and I ∝ 1 / R (for constant applied voltage) as shown in the following circuit diagrams.
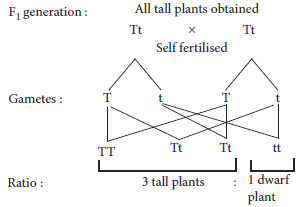
12. In one of his experiments with pea plants Mendel observed that when a pure tall pea plant is crossed with a pure dwarf pea plant, in the first generation, F1 only tall plants appear.
(a) What happens to the traits of the dwarf plants in this case?
(b) When the F1 generation plants were self-fertilised, he observed that in the plants of second generation (F2), both tall plants and dwarf plants were present. Why it happened? Explain briefly.
Ans. (a) Mendel’s monohybrid cross indicated that out of two contrasting traits only one appears in the progeny of first generation. This implies that the trait which appears in F1 generation is dominant and the trait which does not express is recessive. We can also say that gene controlling the dominant trait is dominant gene or allele and gene controlling the recessive trait is recessive gene or allele.
In F1 progeny, although the dominant trait is expressed but genes for both dominant and recessive traits are present in a heterozygous condition. The recessive trait has a chance to express in next generation only if recessive genes come in homozygous condition. This can be illustrated by the given cross:

(b) Appearance of suppressed recessive trait in individuals of F2 generation in Mendelian cross indicates that the characters of recessive traits are not lost even when they are not expressed. When the F1 generation plants were allowed to self-fertilise, both the parental traits were expressed in definite proportion in F2 generation. This could be explained by the given cross by selfing the gametes obtained in F1 generation.
13. Explain the phenomenon of ozone depletion. What are the factors responsible for it. What are its consequences?
Ans. Ozone depletion means the thinning of ozone layer in the atmosphere. Many chemicals mainly chlorofluorocarbons are responsible for ozone depletion. These are widely used as coolants in refrigerators and air conditioners; in fire extinguishers; in aerosol sprayer and as propellants. Once released in the air, these chemicals produce `active chlorine’ (Cl and ClO radicals) in the presence of UV radiations. These radicals, through chain reaction, then destroy the ozone by converting it into oxygen. A single active chlorine can deplete one lakh ozone molecules through chain reaction. Thinning of ozone layer allows ultraviolet (UV) radiations to pass through it which then strike the earth and cause harmful effects on man, animals and plants.
(i) UV radiations increase incidences of skin cancer and herpes.
(ii) UV radiations cause damage to eyes resulting in dimming of eye sight.
(iii) Cause damage to immune system hence, lowering the body’s resistance.
(iv) Harmful UV radiations increase mortality of developing embryo in the mothers, uterus.
(v) These radiations decline the rate of photosynthesis in plants which ultimately increases the CO2 concentration leading to global warming.
SECTION – C
This section has 02 case-based questions (14 and 15). Each case is followed by 03 sub-questions (a, b and c). Parts a and b are compulsory. However, an internal choice has been provided in part c.
14. Andre Marie Ampere suggested that a magnet must exert an equal and opposite force on a current carrying conductor, which was experimentally found to be true. But we know that current is due to charges in motion. Thus, it is clear that a charge moving in a magnetic field experience a force, except when it is moving in a direction parallel to it. If the direction of motion is not parallel to the direction of magnetic field, the magnitude of force experienced depends on the charge, velocity (v), strength of magnetic field (B), and sine of the angle between v and B. Direction of magnetic force is given by Fleming’s left hand rule.
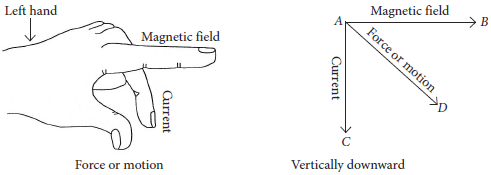
(a) If an electron is travelling horizontally towards east. A magnetic field in vertically downward direction exerts a force on the electron along which direction?
(b) If a charged particle is moving along a magnetic field line. Find the magnetic force on the particle.
(c) A uniform magnetic field exists in the plane of paper pointing from left to right as shown in figure. In the field an electron and a proton are moving as shown. Find the direction of force on electron and proton.
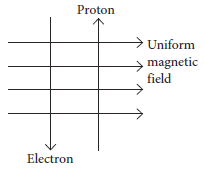
Ans. (a) Fleming’s left hand rule is used to determine the direction of force on electron i.e., in south direction.
(b) The angle between velocity and magnetic field is zero. Therefore, magnetic force on the particle is zero.
(c) As the direction of current is taken opposite to the direction of motion of electrons, therefore, current from the motion of electron and proton is in the same direction, i.e., from bottom to top. Now, according to Fleming’s left hand rule, the electron and the proton experience forces both pointing into the plane of paper.
OR
Why does a current-carrying wire move when placed in magnetic field?
Ans. A current-carrying wire produces a magnetic field around it. When placed in a magnetic field, the two magnetic fields interact with each other and the wire moves.
15. As neutral atom carbon has electronic configuration K L 2, 4 , to gain inert gas configuration it can either donate 4 valence electrons (helium gas configuration) or gain 4 electrons (neon gas configuration), but it cannot do so. To acquire inert gas configuration carbon, can only share its 4 valence electrons with other atoms forming covalent bonds. A covalent bond can be defined as a chemical bond formed between two atoms by mutual sharing of valence electrons so that each atom acquires the stable electronic configuration of the nearest noble gas. The concept of covalent bonds was given by Langmuir and Lewis to explain bonding in non-ionic compounds. The covalent bonds are of three types. If each atom contributes one electron, the covalent bond formed is called a single covalent bond and is represented by a single line (–) and if each atom contributes two electrons, the covalent bond formed is called a double bond and is represented by a double line (=) and if each atom contributes three electrons, the covalent bond formed is called a triple bond and is represented by a triple line (≡).
(a) Out of the compounds, SO2, NH3, HCl and O2, which compounds do not contain a double bond?
(b) Given an example of diatomic molecule containing a triple bond.
(c) Define covalency and give an example of molecule in which all its atoms joined together by double covalent bonds?
Ans. (a) Both NH3 and HCl have single bonds.
(b) N ≡ N or N2 (dinitrogen).
(c) The number of electrons contributed by each atom for sharing is known as covalency. O = C = O or CO2 molecule has all the atoms joined together by double covalent bonds.
OR
Explain briefly the electron dot structure of Cl2 molecule.
Ans. In chlorine molecule, both chlorine atoms contribute one electron and thus share single electron pair to form single covalent bond. As shared pair is shared by both atoms, they acquire inert gas configuration of argon atom in valence shell.