Exam Question for Class 12 Chemistry Chapter 9 Coordination Compounds
Please refer to below Exam Question for Class 12 Chemistry Chapter 9 Coordination Compounds . These questions and answers have been prepared by expert Class 12 Chemistry teachers based on the latest NCERT Book for Class 12 Chemistry and examination guidelines issued by CBSE, NCERT, and KVS. We have provided Class 12 Chemistry exam questions for all chapters in your textbooks. You will be able to easily learn problems and solutions which are expected to come in the upcoming class tests and exams for standard 12th.
Chapter 9 Coordination Compounds Class 12 Chemistry Exam Question
All questions and answers provided below for Exam Question Class 12 Chemistry Chapter 9 Coordination Compounds are very important and should be revised daily.
Very Short Answer Type Questions
Question. When a coordination compound CrCl3⋅6H2O is mixed with AgNO3, 2 moles of AgCl are precipitated per mole of the compound. Write structural formula of the complex.
Answer.For one mole of the compound, two moles of AgCl are precipitated which indicates that two ionisable chloride ions in the complex. Hence, its structural formula is
[CrCl(H2O)5]Cl2.H2O
Question. Explain the term crystal field splitting in an octahedral field.
Answer.The splitting of the degenerate d-orbitals into three orbitals of lower energy, t2g set and two orbitals of higher energy eg set due to the interaction of ligand in an octahedral crystal field is known as crystal field splitting in an octahedral field.

Question. What is the difference between a complex and a double salt?
Answer.Double salts dissociate into ions completely when dissolved in water. On the other hand, in complexes, the complex ion does not dissociate.
Question. Write the formula of the following coordination compound :
Iron (III) hexacyanoferrate(II)
Answer.Fe4[Fe(CN)6]3
Question. Chelates are generally more stable than the complexes of unidentate ligands. Explain.
Answer.Chelates are cyclic compounds so they are more stable than normal complexes. In chelates ligands are held by two or more bonds with the transition metals. e.g.,

Question. What do you understand by ‘denticity of a ligand’?
Answer.Denticity : The number of coordinating groups present in a ligand is called the denticity of ligand.
For example, bidentate ligand ethane-1, 2-diamine has two donor nitrogen atoms which can link to central metal atom.

Question. Write the coordination number and oxidation state of platinum in the complex [Pt(en)2Cl2].
Answer.Coordination number and oxidation state of Pt in the complex [Pt(en)2Cl2] are 6 and +2 because en is a bidentate and neutral ligand.
Question. Write the hybridisation and number of unpaired electrons in the complex [CoF6]3–.(Atomic no. of Co = 27)
Answer.Oxidation state of Co ion in [CoF6]3– is +3.

Question. Why a solution of [Ni(H2O)6]2+ is green while a solution of [Ni(CN)4]2– is colourless?
(At. no. of Ni = 28)
Answer.[Ni(H2O)6]2+ is a high spin complex (Do small) while [Ni(CN)4]2– is a low spin square planar complex.
In [Ni(H2O)6]2+ complex, d-d transitions are taking place on absorbing low energy radiation (red component of spectrum) from visible region showing green as the complementary colour.
In [Ni(CN)4]2– complex, d-d transitions do not take place in the visible region of spectrum, d-d transitions take place in the UV region and hence, complex is colourless.
Question. Write the IUPAC name of [Cr(NH3)6][Co(CN)6].
Answer.Hexaamminechromium(III) hexacyanocobaltate(III).
Short Answer Type Questions (SA-I)
Question. Explain the following term giving a suitable example : Ambidentate ligand
Answer.Ambidentate ligand : A unidentate ligand which can coordinate to central metal atom through two different atoms is called ambidentate ligand.
For example, NO2– ion can coordinate either through nitrogen or through oxygen to the central metal atom/ion.
Question. Out of [CoF6]3– and [Co(C2O4)3]3–, which one complex is
(i) diamagnetic (ii) more stable
(iii) outer orbital complex and
(iv) low spin complex ?
(Atomic no. of Co = 27)
Answer.Formation of [CoF6]3– and [Co(C2O4)3]3– can be represented as :

(i) [Co(C2O4)3]3– is diamagnetic as all electrons are paired.
(ii) [Co(C2O4)3]3– is more stable as C2O42– is a chelating ligand and forms chelate rings.
(iii) [CoF6]3– is an outer orbital complex as it undergoes sp3d2 hybridization using the outer 4d-orbital.
(iv) [Co(C2O4)3]3– is low spin complex due to absence of any unpaired electron.
Question. Write down the IUPAC name of the following complex : [Cr(NH3)2Cl2(en)]Cl
Answer.Diamminedichlorido(ethane-1,2-diamine)chromium(III) chloride.
Question. Give the formula of each of the following coordination entities :
(i) Co3+ ion is bound to one Cl–, one NH3 molecule and two bidentate ethylene diamine (en) molecules.
(ii) Ni2+ ion is bound to two water molecules and two oxalate ions.
Write the name and magnetic behaviour of each of the above coordination entities.
(At. nos. Co = 27, Ni = 28)
Answer.
(i) [Co(en)2Cl(NH3)]2+
Amminechloridobis(ethane-1,2-diamine)cobalt(III) ion
In presence of strong NH3 and en ligand, Co3+ (3d 6) forms low spin complex. Hence, complex is diamagnetic.
(ii) [Ni(ox)2(H2O)2]2– : Diaquadioxalatonickelate(II) ion
In the presence of weak ox and H2O ligand, Ni(II) forms high spin complex (sp3d 2 hybridisation). It is paramagnetic.
Question. Why is [NiCl4]2– paramagnetic but [Ni(CO)4] is diamagnetic? (At. no. : Cr = 24, Co = 27, Ni = 28)
Answer.[NiCl4]2– contains Ni2+ ion with 3d 8 configuration.
Question. Using valence bond theory, explain the geometry and magnetic behaviour of [Co(NH3)6]3+.
(At. no. of Co = 27)
Answer.Oxidation state of cobalt in [Co(NH3)6]3+ is +3.
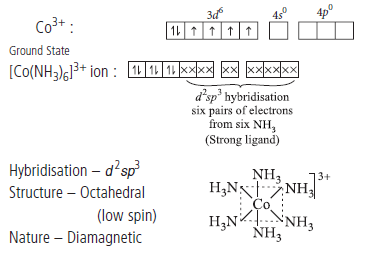
IUPAC name : Hexaamminecobalt(III) ion
Question. (i) On the basis of crystal field theory, write the electronic configuration of d4 ion if Do < P.
(ii) Write the hybridization and magnetic behaviour of the complex [Ni(CO)4].
(At. no. of Ni =28)
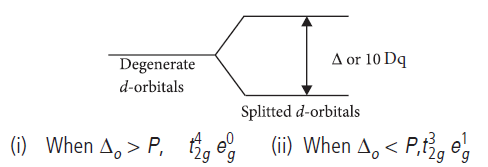
Answer.(i) For d 4 ion, if Δo < P, the fourth electron enters one of the eg orbitals giving the configuration t32g e1g . Ligands for which Δo < P are known as weak field ligands and form high spin complexes.
(ii) [Ni(CO)4] contains Ni(0) – 3d84s2 configuration.
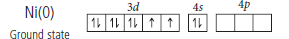
CO is a strong field ligand hence, 4s-electrons will shift to 3d-orbital making 4s-orbital vacant.

The complex has all paired electrons hence, it is diamagnetic.
Question. Ravi prepared a complex compound of cobalt with NH3 and NO2 as donor ligands. He got a red precipitate. Sohan also prepared the same complex using same metal salt solution and same ligands. He obtained yellow crystals.
Sohan complained his teacher that his chemicals were different so he got different product. But their teacher is satisfied with both the results.
Now answer the following questions :
(i) What type of ligand is present in the given compounds which is responsible for changing colour?
(ii) Write IUPAC name of both the compounds.
Answer.
(i) Ambindent ligands (NO2, ONO) present in the given compounds.
(ii) [Co(NH3)5(NO2)]Cl2 (Yellow)
Pentaamminenitrito-N-cobalt(III) chloride
[Co(NH3)5(ONO)]Cl2 (Red)
Pentaamminenitrito-O-cobalt(III) chloride
Question. Why Co2+ is easily oxidised to Co3+ in presence of a strong ligand?
Answer.In presence of strong field ligand, Co(II) has electronic configuration t2g6 eg1.
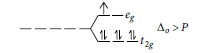
It can easily lose one electron present in eg orbital to give stable t 62g configuration. This is why Co2+ is easily oxidised to Co3+ in the presence of strong field ligand.
Question.Using IUPAC norms write the formulae for the following :
(i) Pentaamminenitrito–O–cobalt(III) chloride
(ii) Potassium tetracyanonickelate(II)
Answer.(i) [Co(NH3)5(ONO)]Cl2 (ii) K2[Ni(CN)4]
Short Answer Type Questions (SA-II)
Question. Name the following coordination entities and describe their structures.
(i) [Fe(CN)6]4–
(ii) [Cr(NH3)4Cl2]+
(iii) [Ni(CN)4]2–
Answer.(i) [Fe(CN)6]4– : Hexacyanidoferrate(II) ion Hybridisation – d 2sp3
Structure : Inner orbital octahedral complex
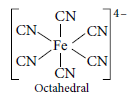
(ii) [Cr(NH3)4Cl2]+ :
Tetraamminedichloridochromium(III) ion
Hybridisation – d 2sp3
Structure : Inner orbital octahedral complex

(iii) [Ni(CN)4]2– : Tetracyanidonickelate(II) ion
Hybridisation – dsp2
Structure – Square planar

Question. For the complex [NiCl4]2–, write
(i) the IUPAC name
(ii) the hybridization type
(iii) the shape of the complex.
(Atomic no. of Ni = 28)
Answer.Tetrachloridonickelate(II) ion
Ni atom (Z = 28)

The complex ion has tetrahedral geometry and is paramagnetic due to the presence of unpaired electrons.
Question. For the complex [Fe(CN)6]3–, write the hybridization type, magnetic character and spin nature of the complex. (At. number : Fe = 26)
Answer.Fe atom (Z = 26)

The complex ion has inner orbital octahedral geometry (low spin) and is paramagnetic due to the presence of one unpaired electron.
Question. (a) What is d-d transition?
(b) Tetrahedral complexes are always of high spin. Explain.
Answer.(a) When ligands approach the central metal, atom or ion of complex its d-orbital breaks into two parts t2g and eg levels. When light falls on the complex the complex absorbs
light of suitable frequency for transfer of electron from lower level to higher level. This jump of electron from one d-level to another is called d-d transition.
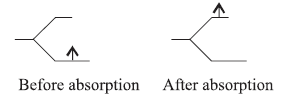
(b) For tetrahedral complexes crystal field splitting energy ∆t is always less than pairing energy. So, tetrahedral complexes are always high spin.
Question. Write the IUPAC names of the following coordination compounds :
(i) [Cr(NH3)3Cl3] (ii) K3[Fe(CN)6]
(iii) [CoBr2(en)2]+
Answer.(i) Triamminetrichloridochromium(III)
(ii) Potassium hexacyanoferrate(III)
(iii) Dibromidobis(ethane-1,2-diamine)cobalt(III) ion
Question. Write the name, the structure and the magnetic behaviour of each one of the following complexes :
(i) [Pt(NH3)2Cl(NO2)] (ii) [Co(NH3)4Cl2]Cl
(iii) Ni(CO)4
(At. nos. Co = 27, Ni = 28, Pt = 78)
Answer.(i) [Pt(NH3)2Cl(NO2)] :
Diamminechloridonitrito-N-platinum(II)
It is square planar and diamagnetic.
(ii) [Co(NH3)4Cl2]Cl :
Tetraamminedichloridocobalt(III) chloride
It is octahedral and diamagnetic.
(iii) Ni(CO)4 : Tetracarbonylnickel(0)
It is tetrahedral and diamagnetic.
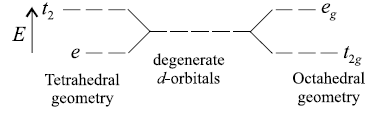
Question. The splitting pattern of d-orbitals in octahedral and tetrahedral geometry are reverse of each other. Why?
Answer.In octahedral complex ligands approach along the axes.
So axial orbitals (dx2 – y2, dz2) lie directly in the path of ligand and experience greater repulsion than the non-axial orbitals. Whereas in tetrahedral complex ligands are closer to nonaxial orbitals (i.e. dxy, dxz and dyz). So, non-axial orbitals experience greater force of repulsion than the axial orbitals.
i.e. approach of ligands in octahedral and tetrahedral fields is opposite of each other. This is why splitting pattern of d-orbitals in octahedral and tetrahedral geometry is reverse of each other.
Question. (a) What is meant by crystal field splitting energy? On the basis of crystal field theory, write the electronic configuration of d4 in terms of t2g and eg in an octahedral field when
(i) Do > P (ii) Do < P
(b) Write two limitations of crystal field theory.
Answer.(a) The difference of energy between two splitted levels of d-orbitals is called crystal field splitting energy. It is denoted by Δ or 10 Dq.
For octahedral Δo, for tetrahedral it is Δt and for square planar Δsp.
(b) (i) It assumes ligand to be point charges.
(ii) It does not take into account the covalent character of bonding between the ligand and the central atom.
Question. For the complex [Fe(en)2Cl2]Cl, identify the following :
(i) Oxidation number of iron
(ii) Hybrid orbitals and shape of the complex
(iii) Magnetic behaviour of the complex
(iv) Name of the complex.
Answer.(i) [Fe(en)2Cl2]Cl
x + 0 × 2 + (–1) × 2 + (–1) × 1 = 0 ∴ x = +3
Oxidation number of iron = +3
(ii) d2sp3 hybridisation and octahedral shape.
(iii) Paramagnetic due to presence of one unpaired electron.
(iv) dichloridobis(ethane-1,2-diamine)iron(III) chloride
Question. Explain the following :
(i) Anhydrous CuSO4 is white while hydrated CuSO4 is blue in colour.
(ii) [Ti(H2O)6]Cl3 is violet in colour but becomes colourless on heating.
Answer.(i) Anhydrous CuSO4 has no ligand. So, crystal field splitting does not occur so, it does not show any colour but in hydrated form it is linked with H2O ligand so, it shows colour due to d-d transition.
(ii) [Ti(H2O)6]Cl3 is a complex compound. In presence of 6 H2O molecules the d-orbitals of Ti3+ undergo splitting. The compound is coloured (violet) due to d-d transition. On heating
water molecules escape, d-orbitals become degenerate. There is no d-d transition. Hence compound becomes colourless.
Question. [Mn(CN)6]3– has two unpaired electrons whereas [MnCl6]3– has four unpaired electrons. Why?
Answer.In [Mn(CN)6]3–, Mn is in +3 state so, it has configuration of 3d4.
Since CN– is a strong field ligand hence pairing of electrons in 3d-orbital takes place.

So, [Mn(CN)6]3– has two unpaired electrons. But in [MnCl6]3–,Cl– is a weak field ligand, so no pairing takes place and it has 4 unpaired electrons.
Question. Write the state of hybridization, the shape and the magnetic behaviour of the following complex entities :
(i) [Cr(NH3)4Cl2]Cl
(ii) [Co(en)3]Cl3
(iii) K2[Ni(CN)4]
Answer.

Question. [Ni(H2O)6]2+ is green and becomes violet when ethane-1, 2-diamine is added to it. Identify the observation.
Answer. Ethane-1,2-diamine is stronger ligand than H2O. When H2O molecule is replaced by ethane-1,2-diamine (en) the crystal field splitting energy (∆) increases. Complex absorbs
light of higher frequency for d-d transition. This is why colour of complex changes from green to violet.
Question. Write the IUPAC name, deduce the geometry and magnetic behaviour of the complex K4[Mn(CN)6].
[Atomic no. of Mn = 25]
Answer.Mn (Z = 25)
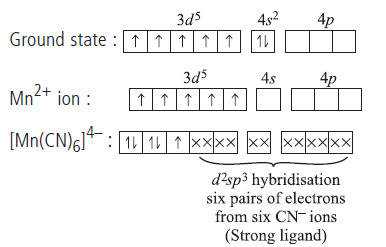
IUPAC name : Potassium hexacyanomanganate(II)
Geometry : Octahedral
No. of unpaired electrons, n = 1
Magnetic behaviour : paramagnetic
Long Answer Type Questions (LA)
Question. Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination
number. Also give stereochemistry and magnetic moment of the complex:
(i) K[Cr(H2O)2(C2O4)2].3H2O
(ii) [Co(NH3)5Cl]Cl2
(iii) [CrCl3(py)3]
(iv) Cs[FeCl4]
(v) K4[Mn(CN)6]
Answer.
(i) K[Cr(H2O)2(C2O4)2].3H2O
Potassium diaquadioxalatochromate(III) trihydrate Oxidation state of Cr = +3; coordination number = 6
Stereochemistry : octahedral
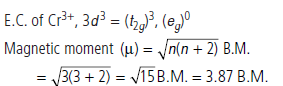
(ii) [Co(NH3)5Cl]Cl2
Pentaamminechloridocobalt(III) chloride
Oxidation state of Co = +3, coordination number = 6
Stereochemistry = octahedral
E.C. of Co3+, 3d6 = (t2g)6, (eg)0
Magnetic moment (µ) = 0
(iii) [CrCl3(py)3]
Trichloridotripyridinechromium(III)
Oxidation state of Cr = +3, coordination number = 6
Stereochemistry : octahedral
E.C. of Cr3+, 3d3 = (t2g)3, (eg)0
Magnetic moment = 3.87 B.M.
(iv) Cs[FeCl4]
Caesium tetrachloridoferrate(III)
Oxidation state of Fe = +3; coordination number = 4
Stereochemistry = Tetrahedral
E.C. of Fe3+, 3d 5 = (e)2, (t2)3
Magnetic moment = 5(5 +2) B.M. = 35 B.M. = 5.92B.M.
(v) K4[Mn(CN)6]
Potassium hexacyanomanganate(II)
Oxidation state of Mn = +2, coordination number = 6
Stereochemistry = octahedral
E.C. of Mn2+, 3d5 = (t2g)5 (eg)0
Magnetic moment

Question. (a) Explain hybridisation in the complex which contains hexacyanidoferrate(III) ion.
(b) Based on the valence bond theory describe the formation and nature of hexaaminecobalt(III) chloride.
(c) How will you show that hexafluorocobaltate(III) ion is paramagnetic in nature?
Answer.
(a) Formation of hexacyanidoferrate(III) ion; [Fe(CN)6]3– :
Electronic configuration of iron in the ground state is 3d 64s2.The oxidation state of iron is +3 in this complex. Iron (III) has outer electronic configuration 3d54s0. It has been experimentally observed that this complex has one unpaired electron.To account for this, two unpaired electrons in 3d subshell pair up, thus leaving two 3d-orbitals empty. These two vacant 3d-orbitals, alongwith one 4s-orbital and three 4p-orbitals hybridise to give six equivalent d2sp3 hybridised orbitals. Six pairs of electrons, one from each cyanide ion, occupy the six vacant hybrid orbitals so produced. The complex has octahedral geometry and is paramagnetic due to the presence of one unpaired electron.
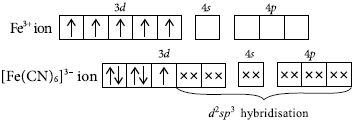
The complex evidently involves (n– 1)d2nsnp3 hybridisation and is, therefore, called inner orbital or low spin complex.
(b) The outer electronic configuration of cobalt (III) ion is 3d6. According to Hund’s rule, four of the 3d-orbitals are singly filled and one 3d-orbital has a pair of electrons.
Octahedral complexes are formed through d2sp3 hybridisation for which the metal atom must have two of its 3d-orbitals empty. This is achieved by the pairing of the two 3d-electrons
as a result of the energy released due to the approach of the ligands. This results in the formation of an octahedral complex. As is evident from the figure, the complex does not contain any unpaired electron and is, therefore, diamagnetic.

Question. (a) What are bidentate ligands? Explain with examples.
(b) Explain the coordination sites of polydentate ligands taking an example of EDTA.
(c) Calculate charge on the central metal in the following complexes:
(i) [Cu(NH3)6]2+ (ii) [Ag(CN)2]–
Answer.(a) Ligands which can coordinate with the central metal atom or ion through two donor atoms are known as bidentate ligands. Examples of bidentate ligands are:

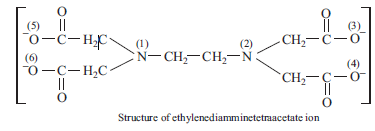
(b) Ligands which coordinate with the central ion through more than two donor atoms present in the molecule are called polydentate ligands. These are called tridentate (three),
tetradentate (four), pentadentate (five) and hexadentate (six) ligands depending upon the number of coordinating donor atoms present in their molecules. A common example of hexadentate ligand is ethylenediamminetetraacetate ion as shown below:
(c) The charge carried by a complex ion is the algebraic sum of charges carried by the central ion and the ligands coordinated to it. Thus [Cu(NH3)6]2+ carries a charge of +2
and because ammonia molecule is neutral therefore, Cu2+ carries a charge of +2. [Ag(CN)2]–, ion carries a charge of –1 and two cyanide ions coordinated to it carry a charge of –1 each. So, Ag+ carries a charge of +1.
Question. (i) Using crystal field theory, draw energy level diagram, write electronic configuration of the central metal atom/ion and determine the magnetic moment value in the following :
(a) [CoF6]3–, (b) [FeF6]3–, (c) [Fe(CN)6]4–
(ii) FeSO4 solution mixed with (NH4)2SO4
solution in 1:1 molar ratio gives the test of Fe2+ ion but CuSO4 solution mixed with aqueous ammonia in 1 : 4 molar ratio does not give the test of Cu2+ ion. Explain why?
Answer.
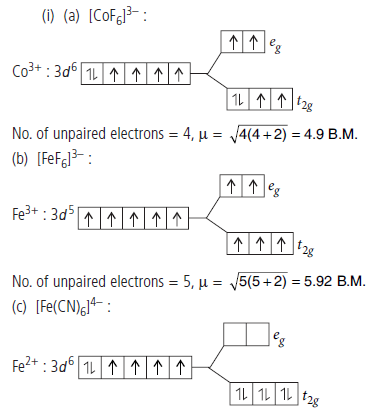
No. of unpaired electrons = 0, m = 0
(ii) When FeSO4 and (NH4)2SO4 solutions are mixed in 1 : 1 molar ratio, Mohr’s salt (a double salt) is formed.

Because Fe2+ ions are formed on dissolution of Mohr’s salt, its aqueous solution gives the test of Fe2+ ions.
When CuSO4 is mixed with ammonia, following reaction occurs :
CuSO4(aq) + 4NH3(aq) → [Cu(NH3)4]SO4
This complex does not produce Cu2+ ion, so the solution of CuSO4 and NH3 does not give the test of Cu2+ ion.
[Cu(NH3)4]SO4 ⇌ [Cu(NH3)4]2+(aq) + SO2–4(aq)
Question. Give reason : [CoF6]3– is outer orbital but [Co(NH3)6]3+ is inner orbital complex.
Answer.In [CoF6]3–, Co is in +3 state and has 3d 6 configuration
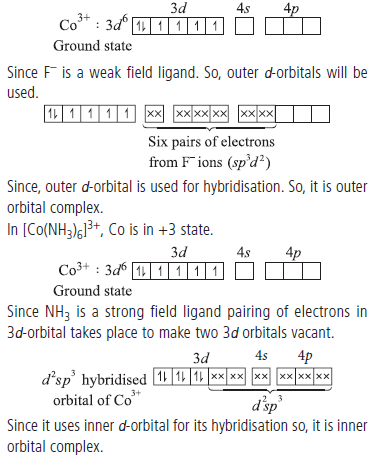
Question. Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complexes :
(i) K3[Co(C2O4)3]
(ii) [Cr(en)2Cl2]Cl
(iii) (NH4)2[CoF4]
(iv) [Mn(H2O)6]SO4
Answer.

