MCQs for Chemistry Class 11 with Answers Chapter 13 Hydrocarbons
Students of class 11 Chemistry should refer to MCQ Questions Class 11 Chemistry Hydrocarbons with answers provided here which is an important chapter in Class 11 Chemistry NCERT textbook. These MCQ for Class 11 Chemistry with Answers have been prepared based on the latest CBSE and NCERT syllabus and examination guidelines for Class 11 Chemistry. The following MCQs can help you to practice and get better marks in the upcoming class 11 Chemistry examination
Chapter 13 Hydrocarbons MCQ with Answers Class 11 Chemistry
MCQ Questions Class 11 Chemistry Hydrocarbons provided below have been prepared by expert teachers of grade 11. These objective questions with solutions are expected to come in the upcoming Standard 11 examinations. Learn the below provided MCQ questions to get better marks in examinations.
Question. The percentage of 1-chloro-2-methylpropane obtained in the chlorination of iso-butane is
(a) 38 %
(b) 64 %
(c) 79 %
(d) 36 %
Answer
B
Question. The trans-alkenes are formed by the reduction of alkynes with
(a) H2-Pd/C, BaSO4
(b) NaBH4
(c) Na/liq. NH3
(d) Sn-HCl
Answer
C
Question. Arrange in the correct order of stability (decreasing order) for the following molecules

(a) (I) > (II) > (III) > (IV)
(b) (IV) > (III) > (II) ≈ (I)
(c) (I) > (II) ≈ (III) > (IV)
(d) (III) > (I) ≈ (II) > (IV)
Answer
D
Question. Butene-1 may be converted to butane by reaction with
(a) Zn-HCl
(b) Sn-HCl
(c) Zn-Hg
(d) Pd/H2
Answer
D
Question. On mixing a certain alkane with chlorine and irradiating it with ultraviolet light, it forms only one monochloroalkane. This alkane would be
(a) propane
(b) pentane
(c) iso-pentane
(d) neo -pentane
Answer
D
Question. The increasing order of reduction of alkyl halides with zinc and dilute HCl is
(a) R—Cl<R —I<R —Br
(b) R—Cl<R—Br<R—I
(c) R—I<R—Br<R—Cl
(d) R—Br<R—I<R—Cl
Answer
B
Question. Which of the following is the most stable conformation of n-butane?
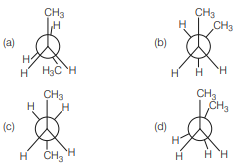
Answer
C
Question. Which one of the following has the minimum boiling point?
(a) n-butane
(b) 1-butyne
(c) 1-butene
(d) Iso -butene
Answer
D
Question. One mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having a molecular mass of 44 u. The alkene is
(a) propene
(b) 1-butene
(c) 2-butene
(d) ethene
Answer
B
Question. A hydrocarbon ‘A’ on ozonolysis gives two isomeric forms ‘B ’ and ‘C ’. B on oxidation gives ‘D’, silver salt of D contains 59.6% Ag. Structure of hydrocarbon is
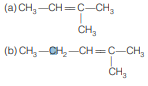
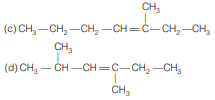
Answer
B
Question. 2-phenylpropene on acidic hydration gives
(a) 2-phenyl propan-2-ol
(b) 2-phenyl propan-1-ol
(c) 3-phenyl propan-1-ol
(d) 1-phenyl propan-2-ol
Answer
A
Question. The number of optically active products obtained from the complete ozonolysis of the given compound is

Answer
A
Question. Arrange the following in the decreasing order of their boiling points.
A. n-butane
B. 2-methylbutane
C. n-pentane
D. 2, 2-dimethylpropane
(a) A > B > C>D
(b) B > C > D>A
(c) D > C > B>A
(d) C > B > D>A
Answer
D
Question. The intermediate formed during the addition of HCl to propene in the presence of peroxide is
(a) CH3CHCH2Cl
(b) CH3CHCH3
(c) CH3CH2CH2
(d) CH3CH2CH2
Answer
B
Question Which of the following compounds is expected to give the highest ratio of ortho/para-isomer (relatively more ortho) when reacted with Cl / FeCl 2 3?

Answer
A
Question. Which of the following reactions will yield 2,2-dibromopropane?
(a) CH3C ≡ CH + 2HBr→
(b) CH3CH = CHBr + HBr 3 →
(c) CH ≡ CH + 2HBr→
(d) CH3CH = CH2 + HBr 3 2 →
Answer
A
Question. The correct sequence of reactions to be performed to convert benzene intom-bromoaniline is
(a) nitration, reduction, bromination
(b) bromination, nitration, reduction
(c) nitration, bromination, reduction
(d) reduction, nitration, bromination
Answer
C
Question. Which compound would give 5-keto-2-methyl hexanal upon ozonolysis?
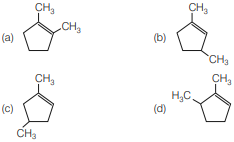
Answer
B
Question. 2-hexyne gives trans-2-hexene on treatment with
(a) Pt /H2
(b) Li/NH3
(c) Pd/BaSO4
(d) LiAlH4
Answer
B
Question. Select the correct statement(s).
(a) Addition of Br − in the following leads to the loss of
resonance energy that is associated with the aromatic ring.

Answer
D
Question. Ozonolysis of an organic compound gives formaldehyde as one of the products. This confirms the presence of
(a) two ethylenic double bonds
(b) a vinyl group
(c) an iso propyl group
(d) an acetylenic triple bond
Answer
B
Question. Pure methane can be produced by
(a) Wurtz reaction
(b) Kolbe’s electrolytic method
(c) sodalime decarboxylation
(d) reduction with H2
Answer
C
Question. Which of the following molecules, in pure form, is (are) unstable at room temperature?

Answer
B
Question. The reaction of propene with HOCl (Cl2 + H2O) proceeds through the intermediate
(a) CH3 —CH —CH2 —Cl
(b) CH3 —CH(OH) —CH3
(c) CH3 —CHCl —CH3
(d) CH3 — CH —CH2 —OH2
Answer
A
Question. Al4C3 on hydrolysis yields
(a) nitrogen gas
(c) hydrogen gas
(b) methane gas
(d) carbon dioxide
Anwser
B
Question. Aqueous solution of an organic compound, A on electrolysis liberates acetylene and CO2 at anode. A is
(a) potassium acetate
(b) potassium succinate
(c) potassium citrate
(d) potassium maleate
Anwser
D
Question. CaC2 + H2O → X →O3/H2O/H+
HCOOH + HCOOH, X is
(a) C2H4
(b) C2H2
(c) C2H6
(d) Ca(OH)2
Anwser
B
Question. Which of the following reagents when heated with ethyl chloride, forms ethylene ?
(a) Aqueous KOH
(b) Zn/HCl
(c) Alcoholic KOH
(d) HI
Anwser
C
Question. When sodium propionate is heated with soda lime, the product formed is
(a) methane
(b) ethane
(c) ethene
(d) ethyne
Anwser
B
Question. The reaction conditions used for converting 1, 2-dibromopropane to propylene are
(a) KOH, alcohol/Δ
(b) KOH, water/Δ
(c) Zn, alcohol/Δ
(d) Na, alcohol/Δ
Anwser
C
Question. By Wurtz reaction, a mixture of methyl iodide and ethyl iodide gives
(a) butane
(b) ethane
(c) propane
(d) a mixture of the above three
Anwser
D
Question. Which one of the following methods is neither meant for the synthesis nor for separation of amines ?
(a) Curtius reaction
(b) Wurtz reaction
(c) Hofmann method
(d) Hinsberg method
Anwser
B
Question. Iso-propyl bromide on Wurtz reaction gives
(a) hexane
(b) propane
(c) 2, 3-dimethyl butane
(d) neo-hexane
Anwser
C
Question. The carbide which reacts with water to form ethyne is
(a) CaC2
(b) SiC
(c) Mg2C3
(d) Al4C3
(e) Be2C
Anwser
A
Question. Which one of the following is the most reactive towards ring nitration?
(a) Benzene
(b) Mesitylene
(c) Toluene
(d) m -xylene
Answer
B
Question. Which one is the most reactive towards electrophilic reagent?

Answer
B
Question. When subjected to acid catalysed hydration, the order of reactivity of the alkenes is
I. (CH3)2C = CH2
II. CH3CH = CH2
III. CH2 = CH2
(a) III > II > I
(b) I > III > II
(c) I > II > III
(d) II > I > III
Answer
C
Question. Acetylene does not react with
(a) Na
(b) ammonical AgNO3
(c) HCl
(d) NaOH
Answer
D
Question. The electrophile E+ attacks the benzene ring to generate the intermediate σ-complex. Of the following, which σ-complex is of lowest energy ?

Answer
B
Question. What is the best way to carry out the following transformation?
1-pentyne→pentanal
(a) HgSO4 /H2SO4
(b) H2 / Lindlar’s catalyst;O3;Zn-H2O
(c) HIO4 /H2O
(d) BH3; H2O2 /NaOH
Answer
D
Question. When 1, 1-dichloropropane and 2, 2-dichloropropane are reacted separately with aq. KOH solution, compounds ‘A’ and ‘B’ are formed. Both ‘A’ and ‘B’ gave the same product ‘C’ on reduction using amalgamated zinc and HCl. Identify C.
(a) Propyl alcohol
(b) Isopropyl alcohol
(c) Propyl chloride
(d) Propane
Answer
D
Question. Given
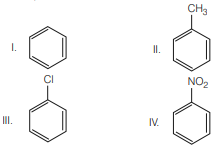
In the above compounds correct order of reactivity in electrophilic substitution reactions will be
(a) II > I > III > IV
(b) IV > III > II > I
(c) I > II > III > IV
(d) II > III > I > IV
Answer
A
Question. 1-butyne on oxidation with hot alkaline KMnO4 would yield
(a) CH3CH2CH2COOH
(b) CH3CH2COOH
(c) CH3CH2COOH + CO2 +H2O
(d) CH3CH2COOH + HCOOH
Answer
C
Question. Give the major product of the following reaction

Answer
B
Question. When propyne is treated with aqueous H2SO4 in presence of HgSO4, the major product is
(a) propanal
(b) n -propyl hydrogen sulphate
(c) acetone
(d) propanol
Answer
C
Question. Two gases P and Q decolourise aqueous bromine but only one of them gives a white precipitate with aqueous ammoniacal silver nitrate solution. P and Q are likely to be
(a) ethane and ethyne
(b) but-1-yne and but-2-yne
(c) ethane and but-2-yne
(d) ethyne and propyne
Answer
B
Question. The addition of HBr to 1-butene gives a mixture of products A, B and C
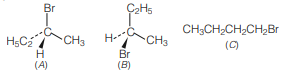
The mixture consists of
(a) A and B as major and C as minor products
(b) B as major, A and C as minor products
(c) B as minor, A and C as major products
(d) A and B as minor and C as major products
Aanswer
A
Question. Ozonolysis of 2,3-dimethyl but-1-ene followed by reduction with zinc and water gives
(a) methanoic acid and 3-methyl butan-2-one
(b) methanal and 2-methyl butan-2-one
(c) methanal and 3-methyl butan-2-one
(d) methanoic acid and 2-methyl butan-2-one
Answer
C
Question. The most stable conformer of 2, 4-hexadiene is
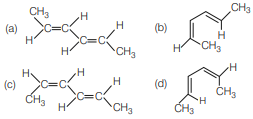
Answer
A
Question. Which of the following is the predominant product in the reaction of HOBr with propene?
(a) 2-bromo-1-propanol
(b) 3-bromo-1-propanol
(c) 2-bromo-2-propanol
(d) 1-bromo-2-propanol
Answer
D
Question. Cyclohexene on ozonolysis followed by reaction with zinc dust and water gives compound E. Compound E on further treatment with aqueous KOH yields compound F. Compound F is
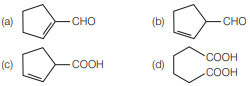
Answer
A
Question. A mixture of ethyl iodide and n-propyl iodide is subjected to Wurtz reaction. The hydrocarbon which will not be formed is
(a) butane
(b) propane
(c) octane
(d) hexane
Answer
B
Question. 6 L of an alkene require 27 L of oxygen at constant temperature and pressure for complete combustion. The alkene is
(a) ethene
(b) propene
(c) 1-butene
(d) 2-butene
Answer
B

We hope the above multiple choice questions for Class 11 Chemistry for Chapter 13 Hydrocarbons provided above with answers based on the latest syllabus and examination guidelines issued by CBSE, NCERT and KVS are really useful for you. Hydrocarbons is an important chapter in Class 11 as it provides very strong understanding about this topic. Students should go through the answers provided for the MCQs after they have themselves solved the questions. All MCQs have been provided with four options for the students to solve. These questions are really useful for the benefits of class 11 students. Please go through these and let us know if you have any feedback in the comments section.
