MCQs for Physics Class 11 with Answers Chapter 13 Kinetic Theory
Students of class 11 Physics should refer to MCQ Questions Class 11 Physics Kinetic Theory with answers provided here which is an important chapter in Class 11 Physics NCERT textbook. These MCQ for Class 11 Physics with Answers have been prepared based on the latest CBSE and NCERT syllabus and examination guidelines for Class 11 Physics. The following MCQs can help you to practice and get better marks in the upcoming class 11 Physics examination
Chapter 13 Kinetic Theory MCQ with Answers Class 11 Physics
MCQ Questions Class 11 Physics Kinetic Theory provided below have been prepared by expert teachers of grade 11. These objective questions with solutions are expected to come in the upcoming Standard 11 examinations. Learn the below provided MCQ questions to get better marks in examinations.
Question. Volume versus temperature graphs for a given mass of an ideal gas are shown in figure at two different values of constant pressure. What can be inferred about relation between P1 and P2 ?
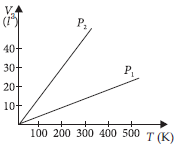
(a) P1 > P2
(b) P1 = P2
(c) P1 < P2
(d) data is insufficient
Answer
A
Question. A gaseous mixture consists of 16 g of helium and 16 g of oxygen. The ratio CP /CV of the mixture is
(a) 1.4
(b) 1.54
(c) 1.59
(d) 1.62
Answer
D
Question. The heat capacity per mole of water is (R is universal gas constant)
(a) 9R
(b) 9/2 R
(c) 6R
(d) 5R
Answer
A
Question. A cylinder containing an ideal M gas is in vertical position and has a piston of mass M that is able to move up or down without friction. If the temperature is increased,

(a) both P and V of the gas will change.
(b) only P will increase according to Charle’s law.
(c) V will change but not P.
(d) P will change but not V.
Answer
C
Question. One kg of a diatomic gas is at a pressure of 8 × 104 N m–2. The density of the gas is 4 kg m–3. What is the energy of the gas due to its thermal motion?
(a) 3 × 104 J
(b) 5 × 104 J
(c) 6 × 104 J
(d) 7 × 104 J
Answer
B
Question. The ratio CP/CV = ϒ for a gas. Its molecular weight is M. Its specific heat capacity at constant pressure is
(a) R / ϒ − 1
(b) ϒR / ϒ − 1
(c) ϒR / M (ϒ − 1)
(d) ϒRM / (ϒ − 1)
Answer
C
Question. If one mole of a monatomic gas {ϒ = 5/3} is mixed with one mole of a diatomic gas {ϒ = 7/3} the value of ϒ for the mixture is
(a) 1.40
(b) 1.50
(c) 1.53
(d) 3.07
Answer
B
Question. Two mole of oxygen is mixed with eight mole of helium. The effective specific heat of the mixture at constant volume is
(a) 1.3R
(b) 1.4R
(c) 1.7R
(d) 1.9R
Answer
C
Question. A gas mixture consists of 2.0 moles of oxygen and 4.0 moles of neon at temperature T. Neglecting all vibrational modes, calculate the total internal energy of the system. (Oxygen has two rotational modes.)
(a) 11 RT
(b) 13 RT
(c) 15 RT
(d) 19 RT
Answer
A
Question. One mole of a monatomic gas is mixed with 3 moles of a diatomic gas. What is the molar specific heat of the mixture at constant volume?
(a) 5 / 4 R
(b) 9 / 4 R
(c) 3 / 4 R
(d) R
Answer
B
Question. Which of the following graphs represent the behaviour of an ideal gas ?
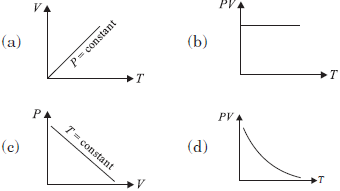
Answer
A
Assertion & Reasoning Based MCQs :
two statements are given-one labelled Assertion (A) and the other labelled Reason (R) Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below.
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true but R is NOT the correct explanation of A
(c) A is true but R is false
(d) A is false and R is also false
Question. Assertion (A) : The root mean square velocity of molecules of a gas having Maxwellian distribution of velocities is higher than their most probable velocity, at any temperature.
Reason (R) : A very small number of molecules of a gas molecules which posses very large velocities.
Answer
C
Question. Assertion (A) : Specific heat of a gas at constant pressure is greater than its specific heat at constant volume.
Reason (R) : At constant pressure, some heat is spent in expansion of the gas.
Answer
A
Question. Assertion (A) : Air pressure in a car tyre increases during driving.
Reason (R) : Absolute zero degree temperature is not zero energy temperature.
Answer
B
Question. Assertion (A) : Vibrational energy of diatomic molecule corresponding to each degree of freedom is kBT.
Reason (R) : For every molecule, vibrational degree of freedom is 2.
Answer
C
Question. Assertion (A) : Absolute zero is not the temperature corresponding to zero energy.
Reason (R) : The temperature at which no molecular motion ceases is called absolute zero temperature.
Answer
A
Question. Assertion (A) : The total translational kinetic energy of all the molecules of a given mass of an ideal gas is 1.5 times the product of its pressure and its volume.
Reason (R) : The molecules of a gas collide with each other and the velocities of the molecules change due to collision.
Answer
B
Question. Assertion (A) : Mean free path of gas molecules varies inversely as density of the gas.
Reason (R) : Mean free path of gas molecules is defined as the average distance travelled by a molecule between two successive collisions.
Answer
B
Question. Assertion (A) : An undamped spring-mass system is simplest free vibration system.
Reason (R) : It has three degrees of freedom.
Answer
C
Question. Assertion (A) : The number of degrees of freedom of a linear triatomic molecules is 7.
Reason (R) : The number of degree of freedom depends on number of particle in the system.
Answer
B
Question. Assertion (A) : The ratio of specific heat of a gas at constant pressure and specific heat at constant volume for a diatomic gas is more than that for a monatomic gas.
Reason (R) : The molecules of a monatomic gas have more degree of freedom than those of a diatomic gas.
Answer
D
Case Based MCQs :
Mean Free Path
The average distance travelled between successive collisions of molecules of a gas is called as mean free path (λ).

Let in time interval t, the molecules moves a distance vt and collides with every molecules in the cylindrical volumes, V = pπd2vt and number of molecules in cylindrical volume be N.
♦ Number of molecules per unit volume be n

Question. The volume of a gas and the number of molecules within that volume for three situations are
(i) 2V0 and N0
(ii) 3V0 and 3N0
(iii) 3V0 and 9N0
The situations are ranked according to the mean free path (greatest first) as
(a) (i), (ii), (iii)
(b) (iii), (ii), (i)
(c) (ii), (iii), (i)
(d) (ii), (i), (iii)
Answer
A
Question. The mean free path of a gas varies with the density of gas according to the following relation

Answer
C
Question. A vessel contains 60,000 molecules of a gas. Due to a very fine hole in the wall, 10,000 molecules escape from the vessel. Then the mean free path of the molecules of the gas
(a) is increased.
(b) is decreased.
(c) is not changed.
(d) may increase or decrease.
Answer
A
Question. The path lengths travelled by a molecule A in 6 collisions are 3, 7, 1, 2, 4, 3 units respectively.
The mean free path of the molecule A is
(a) 13 / 6 unit
(b) 20 / 6 unit
(c) 87 / 6 unit
(d) 6 / 20 unit
Answer
B
Question. Mean free path of a gas molecule is
(a) inversely proportional to number of molecules per unit volume
(b) inversely proportional to diameter of the molecule
(c) directly proportional to the square root of the absolute temperature
(d) directly proportional to the molecular mass
Answer
A
Law of Equipartition of Energy
In equilibrium the total energy is equally distributed in all possible energy modes, with each mode having an average energy equal to (1/2) kT. This is known as law of equipartition energy.
Each translational and rotational degree of freedom contributes (1/2) kT to the energy. Each vibrational frequency contributes 2 × (1/2) kT = kT energy since vibration has both kinetic and potential modes of energy.
Question. The average energy per molecule of a triatomic gas at room temperature T is
(a) 3kT
(b) 1 / 2 kT
(c) 3 / 2 kT
(d) 5 / 2 kT
Answer
A
Question. Which one of the following molecules does not possess vibrational energy?
(a) Oxygen
(b) Nitrogen
(c) Argon
(d) CO2
Answer
C
Question. The gases carbon-monoxide (CO) and nitrogenare kept at the same temperature. If their kinetic energies are E1 and E2 respectively, then
(a) E1 = E2
(b) E1 > E2
(c) E1 < E2
(d) E1 and E2 cannot be compared
Answer
A
Question. According to equipartition law of energy each particle in a system of particles have thermal energy E equal to

Answer
D