Sample Paper Class 12 Chemistry Set D
Please refer to Sample Paper Class 12 Chemistry Set D with solutions provided below. We have provided CBSE Sample Papers for Class 12 Chemistry as per the latest paper pattern and examination guidelines for Standard 12 Chemistry issued by CBSE for the current academic year. The below provided Sample Guess paper will help you to practice and understand what type of questions can be expected in the Class 12 Chemistry exam.
CBSE Sample Paper Class 12 Chemistry for Set D
Topic-1
Classification of Solids Based on Different Binding Forces, Crystal Lattices, Unit Cells,Packing in Solids
Very Short Answer Type Questions
Question. Give an example each of a molecular solid and an ionic solid.
Answer. Examples of Molecular solid : Solid SO2, NH3, I2.
Examples of Ionic solid : NaCl, ZnS, CuCl.
Question. ‘‘Crystalline solids are anisotropic in nature.’’What does this statement mean ?
Answer. It means that some of their physical properties show different electrical and optical properties in different directions in the same crystal.
Question. Write a feature which will distinguish metallic solids from an ionic solid.
Answer. Metallic solids are ductile and malleable whereas ionic solids are not.
Question. How many atoms per unit cell are present in bcc unit cell ?
Answer. 2.
Question. How many atoms constitute one unit cell of a face centred cubic crystal ?
Answer. 4.
Topic-2
Packing Efficiency, Voids, Calculations Related to Unit Cell Dimensions
Very Short Answer Type Questions
Question. What is the formula of a compound in which the element Y forms ccp lattice and atoms of X occupy 1/3rd of tetrahedral voids ?
Answer. Suppose the atoms N in the ccp = n
∴ No. of tetrahedral voids = 2n
No. of atoms M = 2n/3
∴ Ratio of M : N =2n/3: = 2 : 3
Hence, the formula of the compound is M2N3.
Question. What is the formula of a compound in which the element P forms ccp lattice and atoms of Q occupy 2/3rd of tetrahedral voids?
Answer. P3Q4
Question. What is the formula of a compound in which the element P forms hcp lattice and atoms of Q occupy 2/3rd of octahedral voids?
Answer. P3Q2
Question. What is the formula of a compound in which the element P forms ccp lattice and atoms of Q occupy 1/3rd of tetrahedral voids?
Answer. P3Q2
Question. A metallic element crystallises into a lattice having a pattern of AB AB … and packing of spheres leaves out voids in the lattice. What type of structure is formed by this arrangement?
Answer. ccp/fcc
Question. Express the relationship between atomic radius (r) and edge length (a) in the bcc unit cell.
Answer. r =√3a/4
Short Answer Type Questions-I
Question. Silver metal crystallizes with a face-centred cubic lattice. The length of unit cell is found to be 4.077 × 10–8 cm. Calculate atomic radius and density of silver.
(Atomic mass of Ag = 108 u, NA = 6.02 × 1023mol–1).
Answer. Given : a = 4.077 × 10–8 cm, Z = 4, M = 108 u
NA = 6.02 × 1023 mol–1
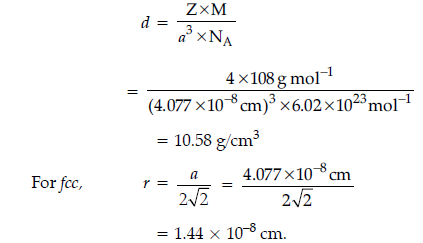
Question. An element with density 2.8 g cm–3 forms a fcc unit cell with edge length 4 × 10–8 cm. Calculate the molar mass of the element. (Given : NA = 6.022 × 1023 mol–1)
Answer. d = 2.8 g cm–3; Z = 4 (for fcc), a = 4 × 10–8 cm; NA
= 6.022 × 1023 mol–1

Question. Niobium crystallizes in body-centred cubic structure. If its density is 8.55 g cm–3, calculate atomic radius of niobium, given its atomic mass 93 u.
Answer.
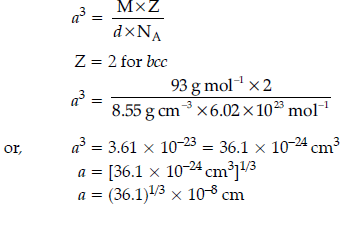
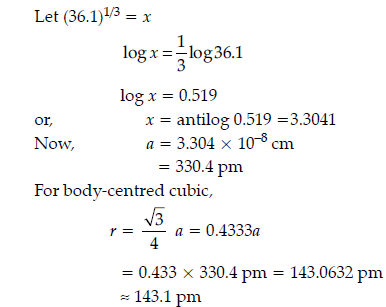
Question. An element crystallizes in a structure having fcc unit cell of an edge 200 pm. Calculate the density if 200 g of this element contains 24 × 1023 atoms.
Answer. Edge length = 200 pm
Volume of the unit cell = (200 × 10–10 cm)3
= 8 × 10–24 cm3
In a fcc unit cell there are four atoms per unit cell.

Question. The unit cell of an element of atomic mass 108 u and density 10.5 g cm–3 is a cube with edge length 409 pm. Find the type of unit cell of the crystal.
(Given : Avogadro’s constant = 6.023 × 1023 mol-1)
Answer.
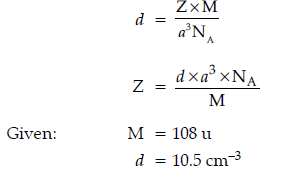
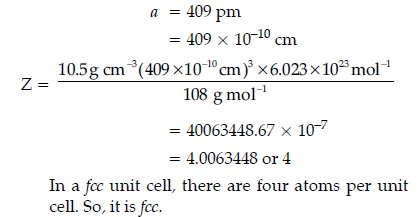
Question. Some of the glass objects recovered from ancient monument look milky instead of being transparent. Why?
Answer. When amorphous solid (glass) are heated and cooled slowly, they acquire crystallinity at same temperature. Glass objects of ancient monuments, over a period of years, are exposed to sunlight and cooled during night period, resulting in the crystallization of the glass object which imparts milky colour to the ancient monuments.
Question. Iron (II) oxide has a cubic structure and each side of the unit cell is 5 Å. If density of the
oxide is 4 g cm–3, calculate the number of Fe2+ and O2– ions present is each unit cell. (Atomic mass Fe = 56 u, Avogadro’s number = 6.023 × 1023 mol–1)
Answer.
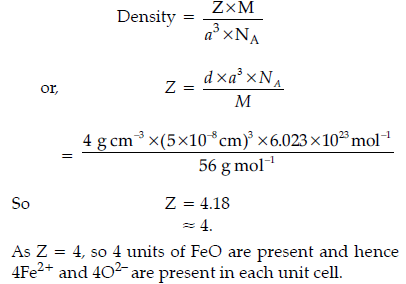
Question. Aluminium crystallizes in a fcc structure. Atomic radius of the metal is 125 pm. What is the length of the side of the unit cell of the metal ?
Answer.

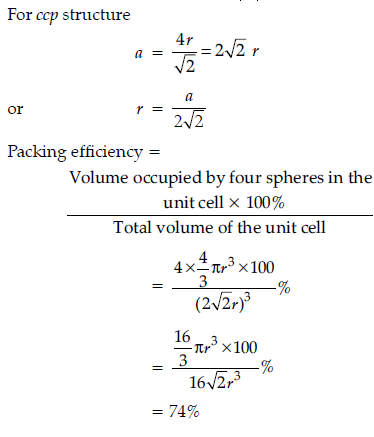
Question. Calculate packing efficiency in ccp structure.
Answer.
Question. If NaCl is doped with 10–3 mole percent SrCl2,what will be the concentration of cation vacancies?
(NA = 6.02 × 1023 mol–1)
Answer. Every Sr2+ ion causes one cation vacancy (because
two Na+ ions are replaced by one Sr2+).
Therefore, introduction of 10–3 moles of SrCl2 per 100 moles of NaCl would introduce 10–3 mole cation vacancies in 100 moles of NaCl.
No. of vacancies per mole of NaCl
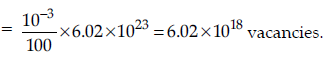
Short Answer Type Questions-II
Question. An element exists in bcc lattice with a cell edge of 288 pm. Calculate its molar mass if its density is 7.2 g/cm3.
Answer.z = 2;
d = (z × M)/a3 × NA (i) 1
Putting values of M in equation (i)
M = 7.2g/cm3 × (288 × 10-10 cm)3 NA/2
= 51.8 g/mol (or any other correct method)
Question. An element crystallises in fcc lattice with cell edge of 400 pm. Calculate its density if 250 g of this element contain 2.5 ´ 1024 atoms.
Answer. In fcc, z = 4;
d = (z × M)/a3 × NA

M = [250 × NA]/2.5 × 1024 (ii)
Putting values of M in equation (i)
d = 4 × 250 g × NA /[2.5 × 1024 atoms × (400 × 10-10 cm3) × NA]
d = 6.25 g/cm3
Question. An element ‘X‘ (At mass = 40 g mol–1) having f.c.c. structure, has unit cell edge length of 400 pm.
Calculate the density of ‘X‘ and the number of unit cells in 4 g of ‘X‘. (NA = 6.022 × 1023 mol–1)
Answer.
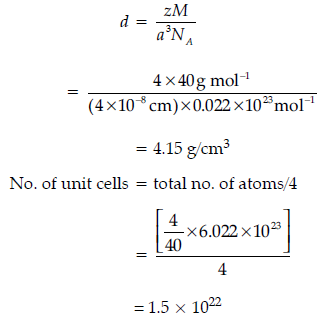
Question. Niobium crystallises in body-centred cubic structure. If the atomic radius is 143.1 pm, calculate the density of Niobium. (Atomic mass = 93 u).
Answer.
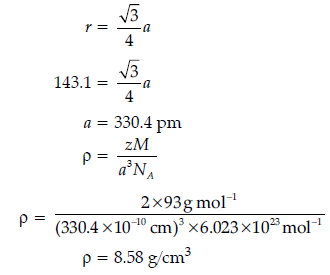
Question. An element crystallizes in a fcc lattice with cell edge of 400 pm. The density of the element is 7 g cm-3. How many atoms are present in 280 g of the element?
Answer.

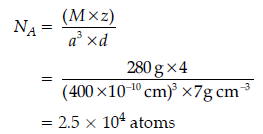
Question. An element crystallises in bcc lattice with cell edge of 400 pm. Calculate its density if 250 g of this element contain 2.5 × 1024 atoms.
Answer.

Question. Silver crystallizes in fcc lattice. If edge length of the unit cell is 4.07 × 10–8 cm and density is 10.5 g cm–3, calculate the atomic mass of silver.
Answer.

Question. Silver crystallizes in fcc lattice. If edge length of the unit cell is 4.077 × 10–8 cm, then calculate the radius of silver atom.
Answer.
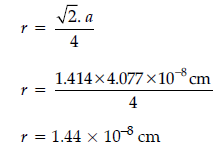
Question. An element with molar mass 27 g mol–1 forms a cubic unit cell with edge length 4.05 × 10–8 cm. If its density is 2.7 g cm–3, what is the nature of the cubic unit cell ?
Answer.
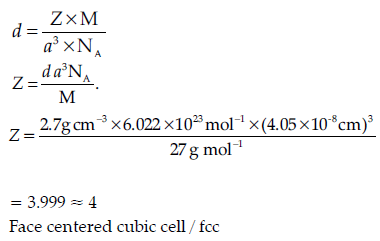
Question. An element occurs in the bcc structure with cell edge of 288 pm. The density of the element is 7.2 g cm–3.How many atoms of the elements does 208 g of the element contain ?
Answer. For the bcc structure, Z = 2

Question.10. An element occurs in the bcc structure with cell edge of 288 pm. The density of the element is 7.2 g cm–3.
How many atoms of the elements does 208 g of the element contain ?
Answer. For the bcc structure, Z = 2Question. Silver crystallizes in face-centred cubic (fcc) unit cell. If the radius of silver atom is 145 pm, what is the length of each side of the unit cell ?
Answer. For fcc cell,
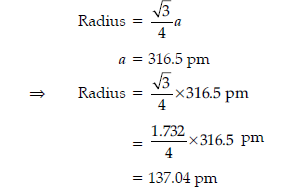
Question. An element occurs in bcc structure. It has a cell edge length of 250 pm. Calculate the molar mass if its density is 8.0 g cm–3. Also calculate radius of an atom of this element.
Answer. a = 250 pm = 250 × 10–10 cm, d = 8 g cm–3, Z = 2
(for bcc), M = ?
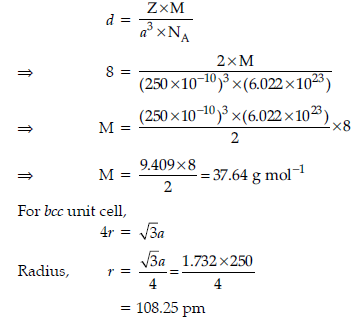
Question. Iron has a body centred cubic unit cell with a cell dimension of 286.65 pm. The density of Iron is 7.874 g cm–3. Use this information to calculate Avogadro’s number. (At mass of Fe = 55.845 u).
Answer.
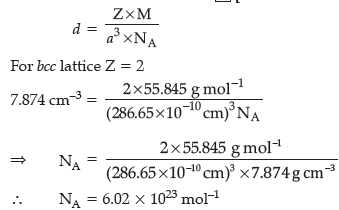
Question. Copper crystallizes with face-centred cubic unit cell. If the radius of copper atom is 127.8 pm,calculate the density of copper metal.
(Atomic mass of Cu = 63.55 g/mol and Avogadro’s number NA = 6.02 × 1023 mol–1)
Answer.

Topic-3
Defects in Solids, Electrical and Magnetic Properties,Band Theory of Metals
Very Short Answer Type Questions
Question. What type of stoichiometric defect is shown by ZnS?
Answer. Frenkel defect.
Question. What type of stoichiometric defect is shown by NaCl?
Answer. Schottky defect.
Question. Which ionic compound shows both Frenkel and Schottky defects?
Answer. AgBr
Question. What type of stoichiometric defect is shown by AgCl? Comptt.
Answer. Frenkel defect.
Question. Name the non-stoichiometric point defect responsible for colour in alkali metal halides.
Answer. Metal excess or anionic vacancies or F-centres.
Question. What type of defect can arise when a solid is heated ?
Answer. Vacancy defect.
Question. What type of stoichiometric defect is shown by AgBr and AgI ?
Answer. Frenkel defect.
Question. Which type of ionic substances show Schottky defect in solids ?
Answer. Schottky defect are more common in ionic compounds with high coordination number and where the sizes of positive and negative ions are almost equal. e.g., NaCl.
Question. Which stoichiometric defect increases the density of a solid ?
Answer. Metal excess defect increase the density of a solid.
Question.On heating a crystal of KCl in potassium vapour, the crystal starts exhibiting a violet colour. What is this due to ?
Answer. This is due to F-centres.
Question. Why does LiCl acquire pink colour when heated in Li vapours ?
Answer. When a crystal of LiCl is heated in an atmosphere of Li vapours, the Li atoms lose electron to form Li+ ions. The released electrons diffuse into the crystal and occupy anionic sites (F-centres). These electrons impart pink colour to the LiCl crystal.
Question. ZnO crystal on heating acquires the formula Zn1+x O. Given reason.
Answer. On heating ZnO, it loses oxygen and there is excess of Zn2+ ions in the crystal.
Question. Analysis shows that FeO has a non-stoichiometric composition with formula Fe0.95O. Give reason.
Answer. Show metal deficiency defect/It is a mixture of Fe2+ and Fe3+/Some ions are replaced by Fe3+/Some of the ferrous ions get oxidised to ferric ions.
Question. What are n-type semiconductors ?
Answer. n-type semiconductors are those substances which have impurity with extra electrons. Example : When silicon doped with group 15th element, n-type semiconductor is obtained.
Question. What is meant by ‘doping’ in a semiconductor ?
Answer. Addition of a suitable impurity to the semiconductor to increase its conductivity is called doping.
Question. How is the conductivity of an intrinsic semiconductor increased ?
Answer. The conductivity is increased by adding an appropriate amount of suitable impurity by doping.
Question. There is an increase in conductivity when silicon is doped with phosphorous. Give reason.
Answer. When silicon is doped with phosphorous (group 15 element), the increase in conductivity is due to the delocalised negatively charged electrons.
Question. What type of substances would make better permanent magnets, ferromagnetic or ferrimagnetic ?
Answer. Ferromagnetic substances.
Question. What type of magnetism is shown by a substance if magnetic moments of domains are arranged in same direction ?
Answer. Ferromagnetism.
Short Answer Type Questions-I
Question. Explain the following terms with suitable examples :
(i) Frenkel defect
(ii) F-centres
Answer. (i) Frenkel defect : The defect in which the smaller ion/cation is dislocated to interstitial site.
Example : Silver halides, ZnS. (Any one)
(ii) F-centres : The anion vacancy occupied by an electron.
Example : NaCl, KCl, LiCl
Question. How will you distinguish between the following pairs of terms :
(i) Tetrahedral and Octahedral voids.
(ii) Crystal lattice and Unit cell.
Answer. (i) Tetrahedral void is surrounded by 4 constituent particles (atoms/molecules/ions).
Octahedral void is surrounded by 6 constituent particles (atoms/molecules/ions).
OR
Radius ratio (r+/r–) for tetrahedral void is 0.225 & radius ratio for octahedral voids is 0.414.
(ii) A regular three dimensional arrangement of points in space is called a crystal lattice.
Unit cell is the smallest portion of a crystal lattice which, when repeated in three directions, generates an entire lattice/unit cell is the miniature of crystal lattice/microscopic edition of the crystal lattice.
Question. (i) Write the type of magnetism observed when the magnetic moments are aligned in parallel and anti-parallel directions in unequal numbers.
(ii) Which stoichiometric defect decreases the density of the crystal ?
Answer. (i) Ferrimagnetism
(ii) Schottky defect
Question. (i) What type of non-stoichiometric point defect is responsible for the pink colour of LiCl ?
(ii) What type of stoichiometric defect is shown by NaCl ?
Answer. (i) Excess lithium makes LiCl crystal pink. It is caused by metal excess defect due to anionic vacancies(F-centres)
(ii) NaCl shows Schottky defect.
Question. (i) Write the type of magnetism observed when the magnetic moments are oppositely aligned and cancel out each other.
(ii) Which stoichiometric defect does not change the density of the crystal ?
Answer. (i) Anti ferromagnetism.
(ii) Frenkel defect.
Question. Examine the given defective crystal
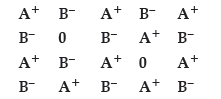
Answer the following questions :
(i) What type of stoichiometric defect is shown by the crystal ?
(ii) How is the density of the crystal affected by this defect ?
(iii) What type of ionic substances show such defect ?
Answer. (i) Schottky defect.
(ii) Decreases
(iii) Alkali metal halides/ionic substances having almost similar size of cations and anions (NaCl/KCl).
Question. Account for the following :
(i) Schottky defects lower the density of related solid.
(ii) Conductivity of silicon increases on doping it with phosphorus.
Answer. (i) In Schotty defect, as the number of ions decreases and volume remains same, so the density decreases.
(ii) This is due to availability of one extra electron provided by P.
Question. (i) Why does presence of excess of lithium makes LiCl crystals pink ?
(ii) A solid with cubic crystal is made of two elements P and Q. Atoms of Q are at the corners of the cube and P at the body centre. What is the formula of the compound ?
Answer. (i) When a crystal of LiCl is present in excess of lithium, the lithium atom loses an e– to from Li+ ions. The released e– diffuse into the crystal and occupies the vaccant anionic sites (F-centre).
These e– imparts the pink colour to the crystal.
(ii) We know that in simple cubic number of atoms at corner 1/8× 8 = 1, contribution of corner = 1. So the formula of compound is PQ.
Question. (i) What change occurs when AgCl is doped with CdCl2 ?
(ii) What type of semiconductor is produced when silicon is doped with boron ?
Answer. (i) Cationic vacancy is generated.
(ii) p-type semiconductor.
Question. Explain the following terms with suitable
examples : Ferromagnetism and Ferrimagnetism.
Answer. Ferromagnetism : (i) Substance which are attracted most easily in magnetic field is called ferromagnetic substance.
(ii) Examples : Fe, Co, Ni, CrO2 and Alnico (alloy of Al, Ni and Co.
(iii) It occurs due to alignment of all magnetic moments (due to unpaired e–) in the same direction.
Ferrimagnetism : (i) A substance which is weakly attracted by the magnetic field is called ferrimagnetic substances.
(ii) Examples : Fe3O4, ferrites having the formula M2+Fe2O4. (where M2+= Cu2+ or Zn2+).
(iii) In these substances, the alignment of magnetic moment in opposite direction are in unequal numbers. (Any two differences)
Question. What is a semiconductor ? Describe the two main types of semiconductors ?
Answer. Semiconductor : A material whose electrical conductivity is of the order of 10–6 – 104 ohm–1 m–1 is called a semiconductor. It allows flow of current only in one direction.
There are two types of semiconductors :
(i) n-type semiconductor : Semiconductors formed after doping with electron rich impurities to increase their conductivity.
(ii) p-type semiconductor : Semiconductors formed by doping with electron deficient impurities to increase their conductivity.
Short Answer Type Questions-II
Question. Examine the given defective crystal :
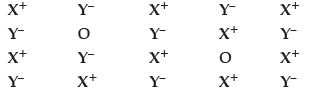
Answer the following questions :
(i) Is the above defect stoichiometric or nonstoichiometric ?
(ii) Write the term used for this type of defect. Give an example of the compound which shows this type of defect.
(iii) How does this defect affect the density of the crystal ?
Answer. (i) Stoichiometric defect.
(ii) Schottky defect NaCl (or any other example).
(iii) Density of crystal decreases.
Question. Define the following :
(i) Schottky defect
(ii) Frenkel defect
(iii) F-centre
Answer. (i) The defect in which equal number of cations and anions are missing from the lattice.
(ii) Due to dislocation of smaller ion from its normal site to an interstitial site.
(iii) Anionic vacancies are occupied by unpaired electron.
Question. (a) Based on the nature of intermolecular forces, classify the following solids: Benzene, Silver
(b) AgCl shows Frenkel defect while NaCl does not.
Give reason.
(c) What type of semiconductor is formed when Ge is doped with Al?
Answer. (a) Benzene – molecular solid
Silver – metallic solid
(b) Size of Ag+ ion is smaller than Na+ ion.
(c) p-type
Question. (i) Based on the nature of intermolecular forces,classify the following solids: Silicon carbide,Argon
(ii) ZnO turns yellow on heating. Why?
(iii) What is meant by groups 12-16 compounds? Give an example?
Answer. (i) Covalent solid/network solid, molecular solid

Because excess Zn2+ ions move to interstitial sites and the electrons move to neighbouring voids
(iii) Compounds prepared by combination of groups 12 and 16 behave like semiconductors.
For e.g., ZnS, CdS, CdSe, HgTe (Any one)
Question. (i) Based on the nature of intermolecular forces, classify the following solids: Sodium sulphate, Hydrogen
(ii) What happens when CdCl2 is doped with AgCl?
(iii) Why do ferrimagnetic substances show better magnetism than antiferromagnetic substances?
Answer. (i) Na2SO4 : Ionic, H2 : Molecular
(ii) Impurity defect/Non-stoichiometric defect
(iii) In ferrimagnetism, domains/magnetic moments are aligned in opposite direction in unequal numbers while in antiferromagnetic the domains align in opposite direction in equal numbers so they cancel magnetic moments completely, net magnetism is zero/ diagrammatic explanation.
Question. (i) What are intrinsic semiconductors ? Give an example.
(ii) What is the distance between Na+ and Cl– ions in NaCl crystal if its density is 2.165 g cm–3 ?
[Atomic Mass of Na = 23u, Cl = 35.5 u; Avogadro’s number = 6.023 × 1023]
Answer. (i) Intrinsic semiconductors are the purest form of semiconductors without any impurities. The most common examples of the intrinsic semiconductors are silicon and germanium.


Long Answer Type Questions
Question. (i) An element has atomic mass 93 g mol–1 and density 11.5 g cm–3. If the edge length of its unit cell is 300 pm, identify the type of unit cell.
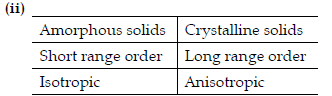
(ii) Write any two differences between amorphous solids and crystalline solids.
OR
(i) Calculate the number of unit cells in 8.1 g of aluminium if it crystallizes in a fcc structure.
(Atomic mass of Al = 27 g mol–1)
(ii) Give reasons:
(a) In stoichiometric defects, NaCl exhibits Schottky defect and not Frenkel defect.
(b) Silicon on doping with Phosphorous forms n-type semiconductor.
(c) Ferrimagnetic substances show better magnetism than antiferromagnetic substances.
Answer.

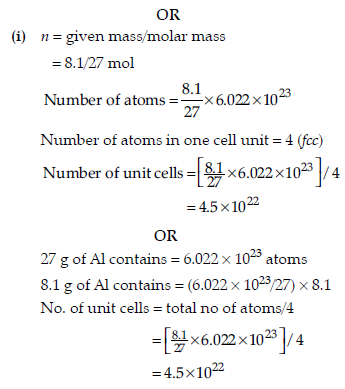
(ii) (a) Due to comparable size of cation and anion/ large size of sodium ion.
(b) p has 5 valence e–, an extra electron results in the formation of n-type semiconductor.
(c) In ferrimagnetism domains/magnetic moments are aligned in opposite direction in unequal numbers while in antiferromagnetic the domains align in opposite direction in equal numbers so they cancel magnetic moments completely, net magnetism is zero/ diagrammatic representation
Question. (i) (a) Following is the schematic alignment of magnetic moments :

What type of magnetism is shown by this substance ?
(b) What type of stoichiometric defect is shown by (i) KCl (ii) AgCl ?
(ii) An element with density 11.2 g cm–3 forms a fcc lattice with edge length of 4 × 10–8 cm.
Calculate the atomic mass of the element.(NA = 6.02 × 1023 mol–1)
OR
Silver metal crystallises with a face centred cubic lattice. The length of the unit cell is found to be 3.0 × 10–8 cm. Calculate atomic radius and density of silver.
(Molar mass of Ag = 108 g mol–1, NA = 6.02 × 1023 mol–1).
Answer. (i) (a) Antiferromagnetism
(b) (i) Schottky defect (ii) Frenkel Deffect

Question. (i) Following is the schematic alignment of magnetic moments:
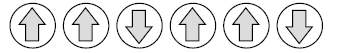
Identify the type of magnetism. What happens when these substances are heated?
(ii) If the radius of the octahedral void is ‘r’ and radius of the atoms in close packing is ‘R’. What is the relation between ‘r’ and ‘R’?
(iii) Tungsten crystallizes in body centred cubic unit cell. If the edge of the unit cell is 316.5 pm. What is the radius of Tungsten atom?
OR
(i) Identify the type of defect shown in the figure:
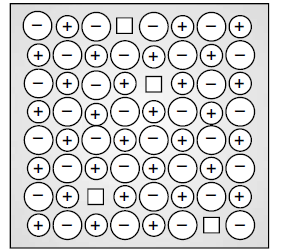
What type of substances show this defect?
(ii) A metal crystallizes in a body centred cubic structure. If ‘a’ is the edge length of its unit cell, ‘r’ is the radius of the sphere. What is the relationship between ‘r’ and ‘a’?
(iii) An element with molar mass 63 g/mol forms a cubic unit cell with edge length of 360.8 pm. If its density is 8.92 g/cm3. What is the nature of the cubic unit cell?
Answer. (i) Ferrimagnetism.
These substances lose ferrimagnetism on heating and become paramagnetic.
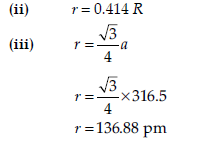
OR
(i) Schottky defect
It is shown by ionic substances in which the cation and anion are of almost similar sizes.
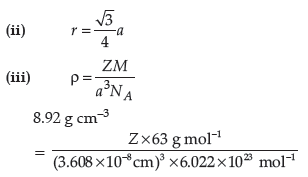
Z = 4. So it is face centred cubic lattice
